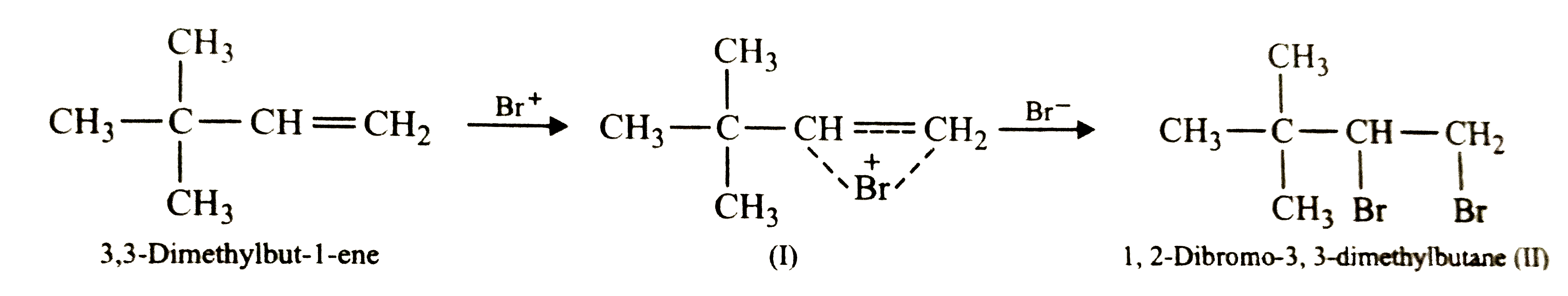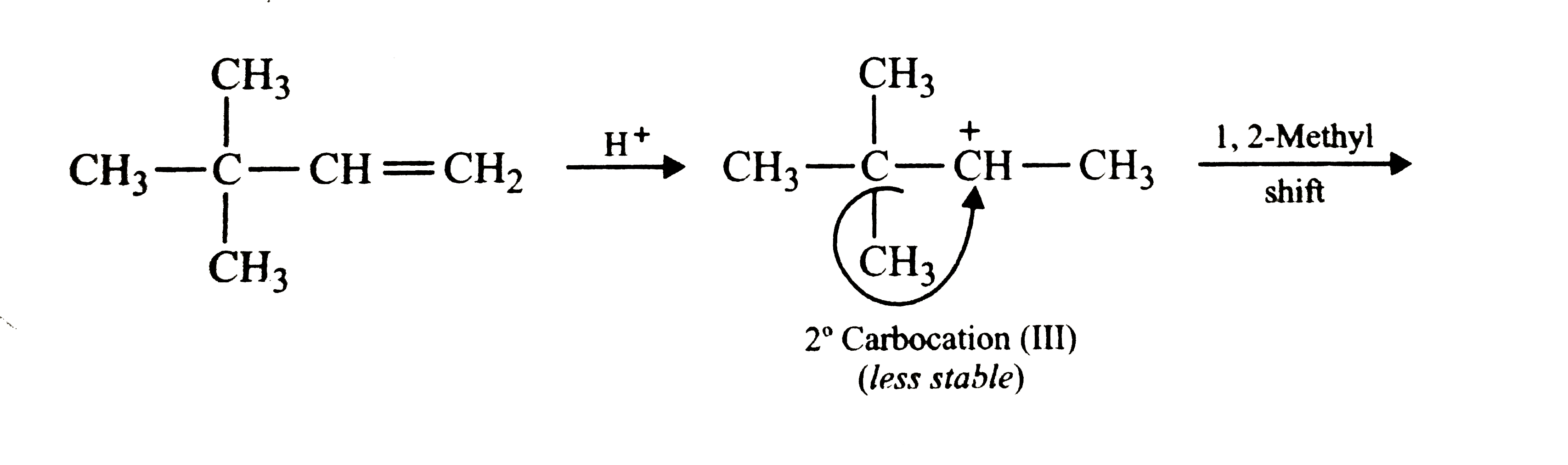Text Solution
Verified by Experts
Topper's Solved these Questions
HYDROCARBONS
PRADEEP|Exercise ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS (PROBLEMS)|2 VideosHYDROCARBONS
PRADEEP|Exercise Competition Focus (JEE(main and advanced)/Medical Entrance) I. MULTIPLE CHOICE QUESTIONS|189 VideosHYDROCARBONS
PRADEEP|Exercise ADDITIONAL QUESTIONS (LONG ANSWER QUESTIONS)|11 VideosEQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSES
PRADEEP|Exercise Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)|10 VideosHYDROGEN
PRADEEP|Exercise COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2|15 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-HYDROCARBONS-ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS (QUESTIONS)
- vdisplayoptions
Text Solution
|
- n-Butane prepared by Wurtz reaction of ethyl bromide is always contam...
Text Solution
|
- Methyl chloride prepared by chlorination of methane is always contamin...
Text Solution
|
- tert-Butyl chloride on treatment with LiAlH4 gives 2-methylpropene but...
Text Solution
|
- Hydrocarbon (A) reacts with bromine by substitution to form an alkyl b...
Text Solution
|
- Four aromatic carboxylic acids on heating with soda-lime at 630 K, giv...
Text Solution
|
- Generally the melting points of isomeric alkanes decreases with branch...
Text Solution
|
- Addition of HBr to 2-butene gives two products while that of HBr to 1...
Text Solution
|
- Explain why the addition of HI to 3,3-dimethylbut-1-ene gives 2-iodo-2...
Text Solution
|
- Addition of H2 to 2-butyne in presence of Lindlar's catalyst . Gives c...
Text Solution
|
- Which of the two : trans-but-2-ene-or trans- pent-2- ene is non polar ...
Text Solution
|
- Why HF forms H-bonding with ethyne even though it is non-polar in natu...
Text Solution
|
- Which of the following has higher dipole moment and why? But -1- ene...
Text Solution
|
- A and B are two salts. A reacts both with dil H2SO4 and conc. H2SO4 to...
Text Solution
|
- Benzenesulphonic acid when heated with steam gives benzene but nitrobe...
Text Solution
|
- Consider the following molecules : Compound (I) is aromatic while...
Text Solution
|