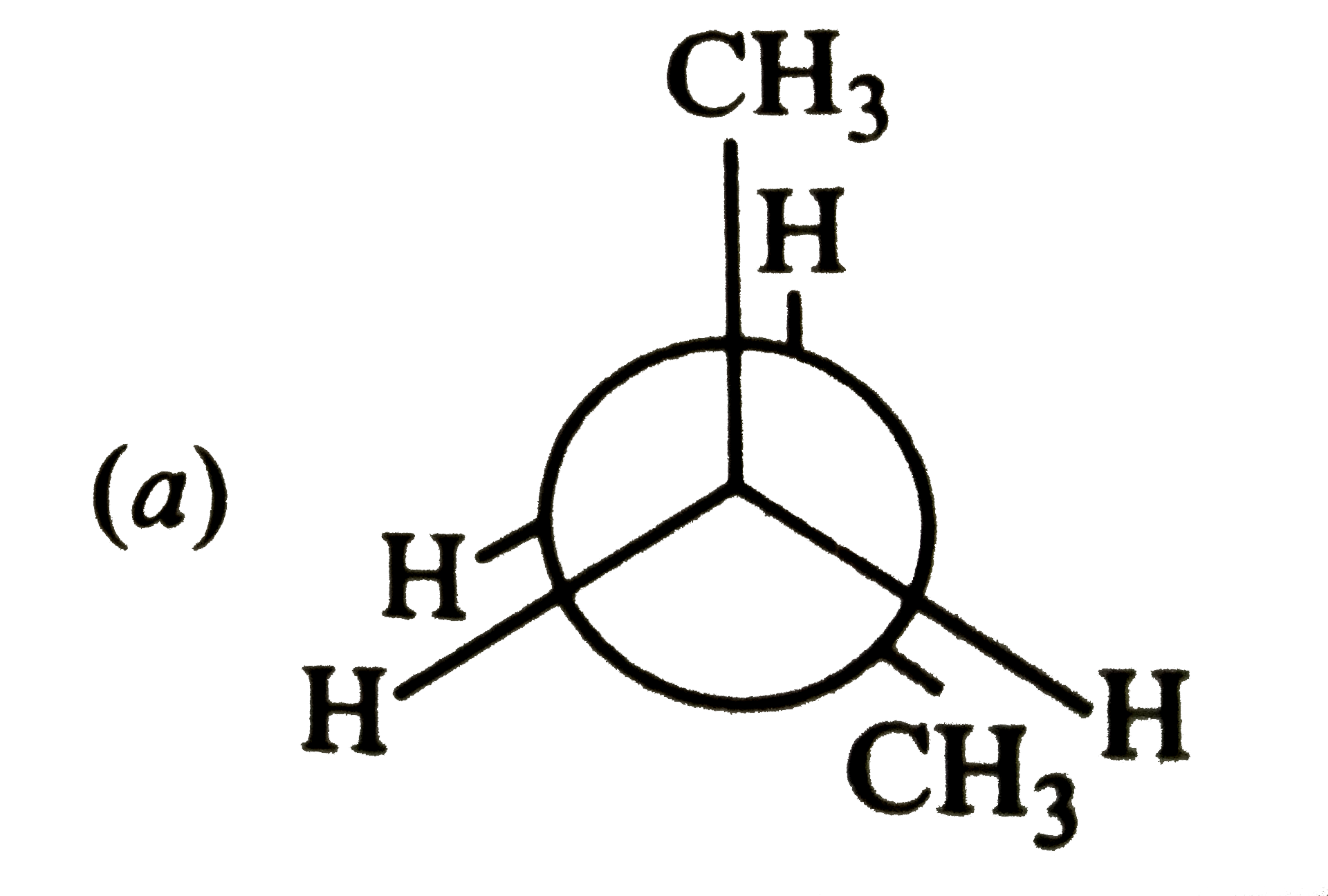
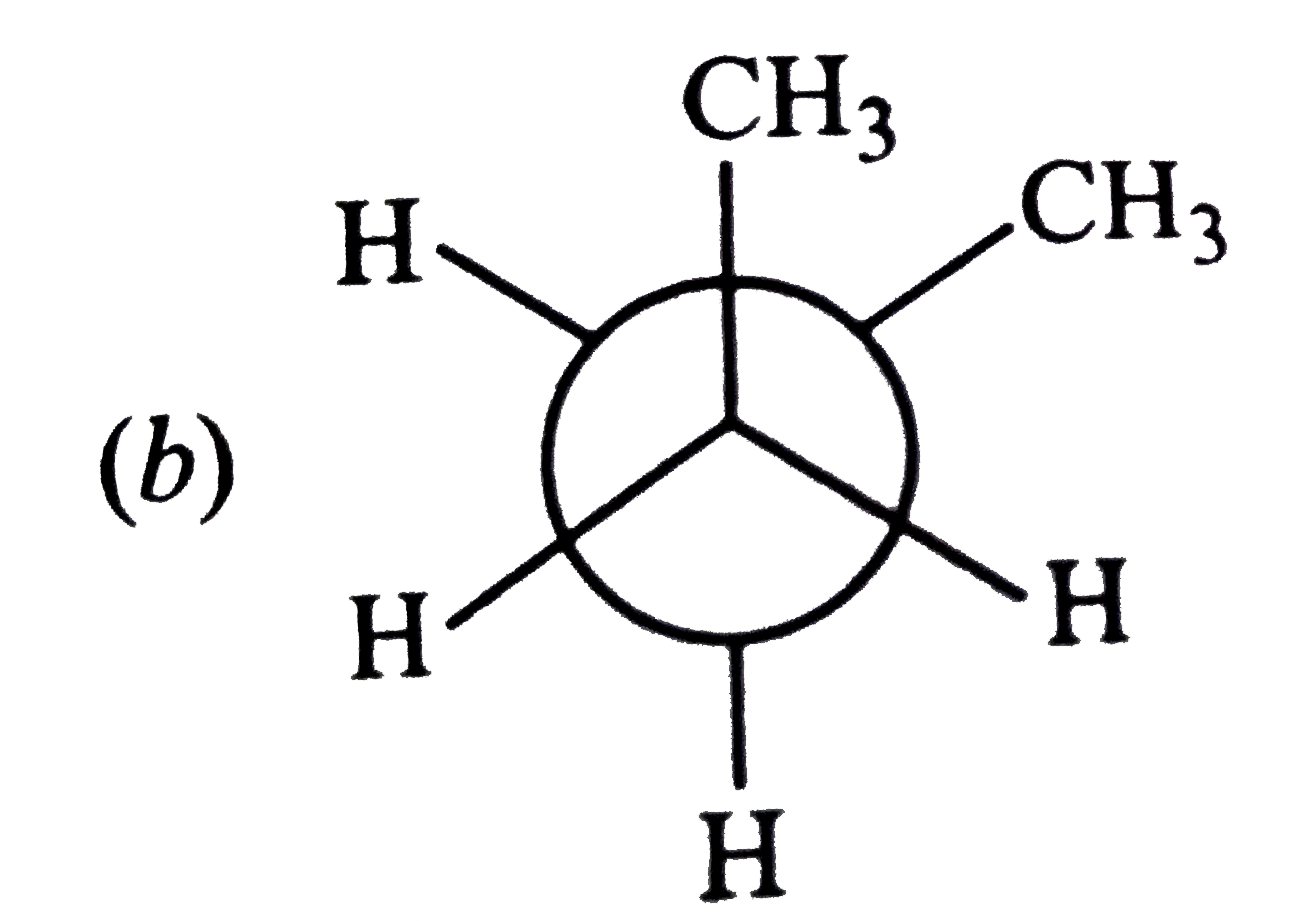
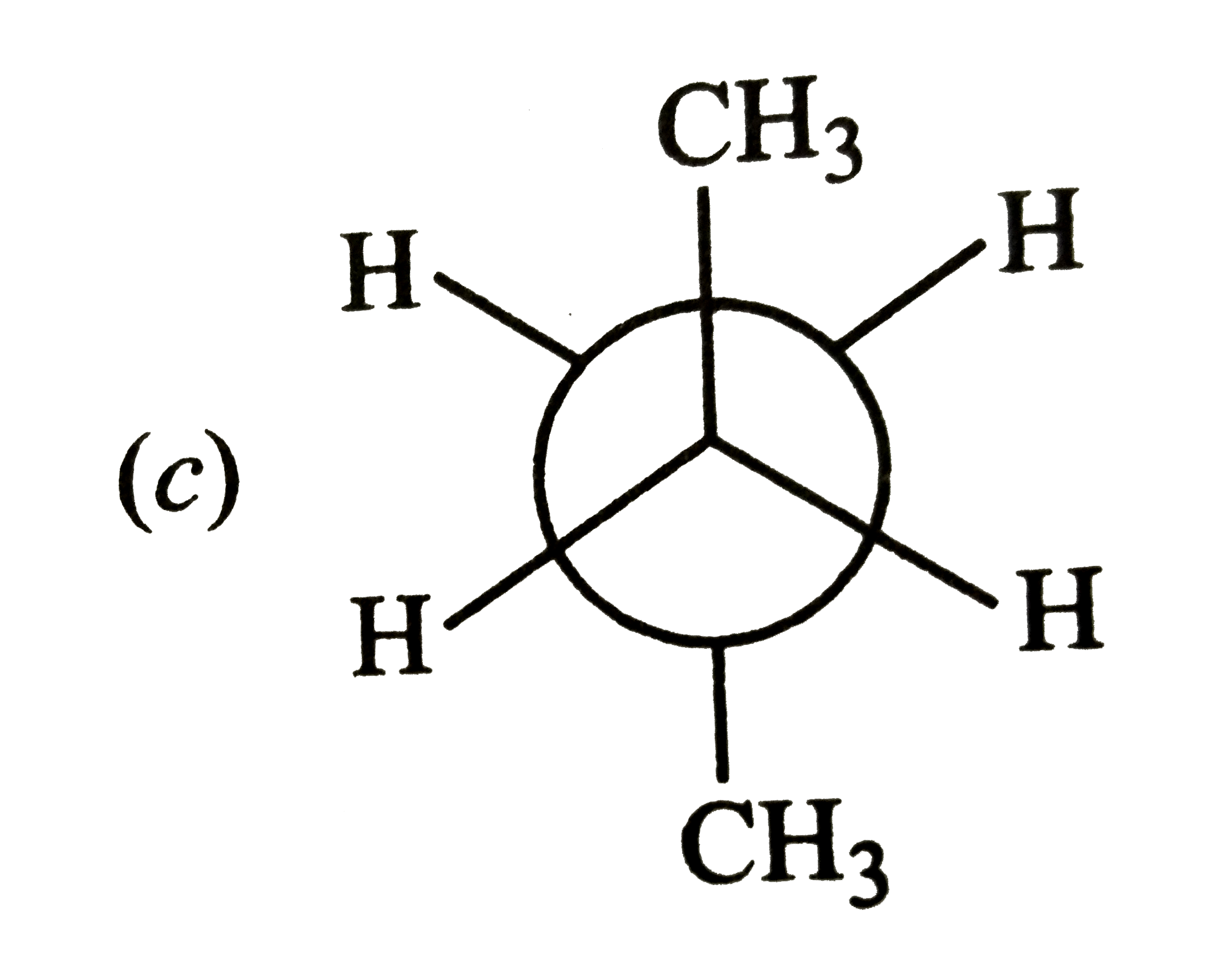
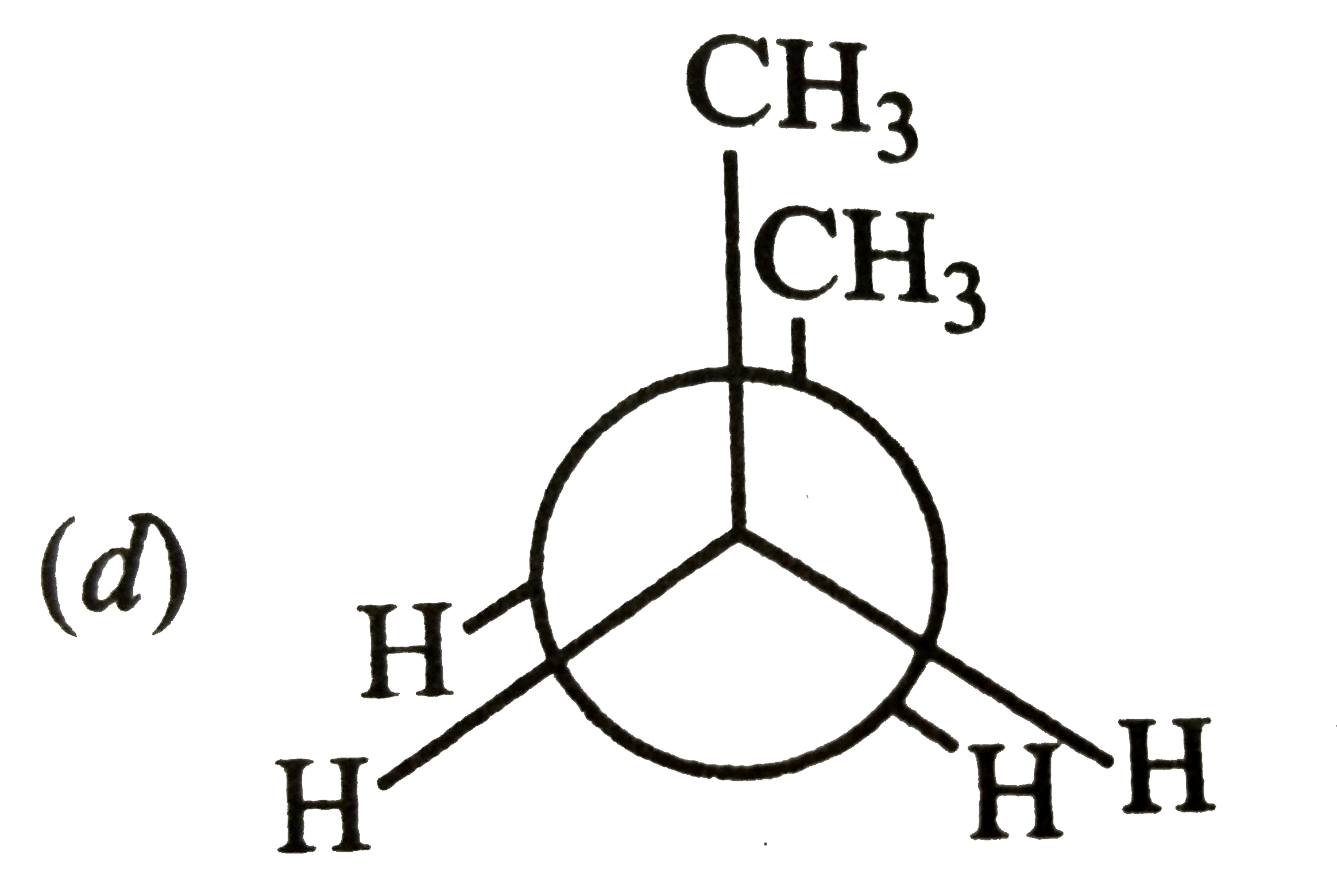
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
PRADEEP|Exercise Competition Focus (JEE(main and advanced)/Medical Entrance) II. MULTIPLE CHOICE|14 VideosHYDROCARBONS
PRADEEP|Exercise Competition Focus (JEE(main and advanced)/Medical Entrance) III. MULTIPLE CHOICE|14 VideosHYDROCARBONS
PRADEEP|Exercise ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS (PROBLEMS)|2 VideosEQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSES
PRADEEP|Exercise Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)|10 VideosHYDROGEN
PRADEEP|Exercise COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2|15 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-HYDROCARBONS-Competition Focus (JEE(main and advanced)/Medical Entrance) I. MULTIPLE CHOICE QUESTIONS
- Increasing order of stability among the three main conformation ( i.e....
Text Solution
|
- Which of the following conformers for ethylene glycol is most stable?
Text Solution
|
- Among the following , the most stable conformation of n-butane is
Text Solution
|
- The correct order of decreasing H-C-H angle in the following molecule ...
Text Solution
|
- Which of the following compounds will exhibits geometrical isomerism ?
Text Solution
|
- Which of the following exhibits geometrical isomerism ?
Text Solution
|
- The IUPAC name of the following compound is
Text Solution
|
- Which of the following compounds exhibits geometrical isomerism ?
Text Solution
|
- Geometrical isomerism is not possible in
Text Solution
|
- The number of isomers for the compound with molecular formula C(2)BrCl...
Text Solution
|
- The Z-isomer among the following is
Text Solution
|
- The major product formed when 2-bromo-2-methylbutane is refluxed with ...
Text Solution
|
- Which of the following organohalogen compound when heated with alcohol...
Text Solution
|
- Which of the following compounds shall not produce propene by reaction...
Text Solution
|
- CH(3)CH(2)-underset(CH(3))underset(|)overset(CH(3))overset(|)(C)-under...
Text Solution
|
- In the reaction below, X is Neopentyl alcohol overset(H(2)SO(4))rarr...
Text Solution
|
- The main product of the following reaction is C6H5CH2CH(OH)CH(CH3)2 ov...
Text Solution
|
- Cyclohexene is best prepared from cyclohexanol by which of the followi...
Text Solution
|
- Which of the following is not the product of dehydration of
Text Solution
|
- The compound that will react most readily with gaseous bromine has the...
Text Solution
|