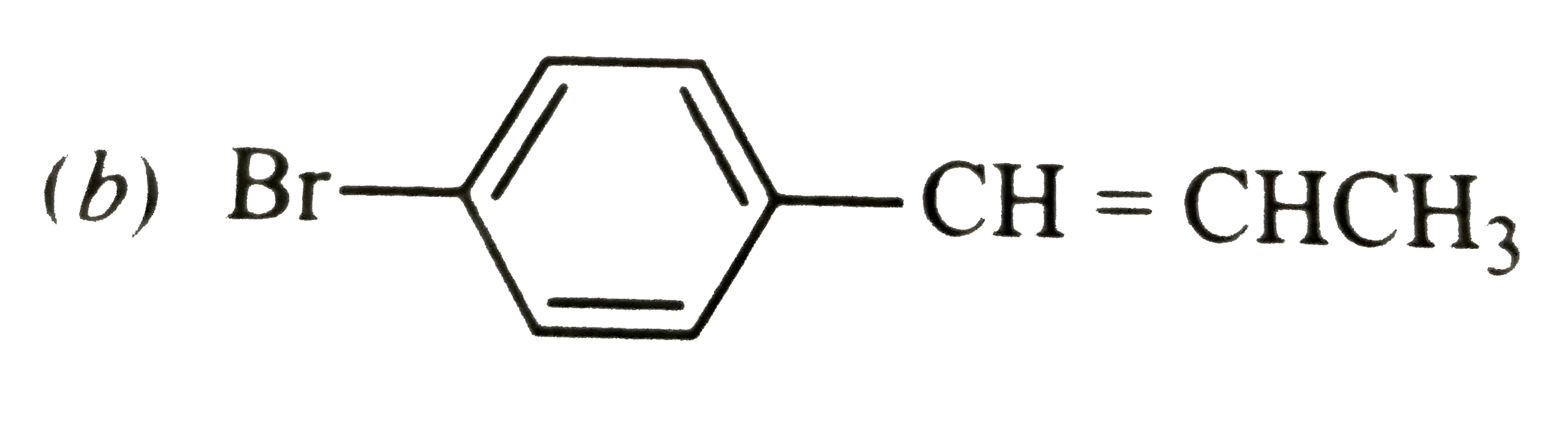
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
PRADEEP|Exercise Competition Focus (JEE(main and advanced)/Medical Entrance) II. MULTIPLE CHOICE|14 VideosHYDROCARBONS
PRADEEP|Exercise Competition Focus (JEE(main and advanced)/Medical Entrance) III. MULTIPLE CHOICE|14 VideosHYDROCARBONS
PRADEEP|Exercise ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS (PROBLEMS)|2 VideosEQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSES
PRADEEP|Exercise Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)|10 VideosHYDROGEN
PRADEEP|Exercise COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2|15 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-HYDROCARBONS-Competition Focus (JEE(main and advanced)/Medical Entrance) I. MULTIPLE CHOICE QUESTIONS
- The compound that will react most readily with gaseous bromine has the...
Text Solution
|
- 3-Phenylpropene on reaction with HBr gives (as major product)
Text Solution
|
- The reaction of C6H5CH=CHCH3 with HBr produces
Text Solution
|
- H3C-undersetunderset(CH3)|CH-CH=CH2+HBr to A A (predominantly ) is
Text Solution
|
- HBr reacts with CH2=CH-OCH3 under anhydrous condditions at room temper...
Text Solution
|
- The additon of HBr to 2-pentene gives
Text Solution
|
- In the reaction with HCl, an alkene reacts in accordance with Markowni...
Text Solution
|
- In which of the following can peroxide effect operate ?
Text Solution
|
- The alkene that will give the same product with HBr in the presence as...
Text Solution
|
- CH3CH2-CH=CH2+HBr overset"ROOR"to underset"Major"[X]+underset"Minor"[Y...
Text Solution
|
- The reaction of propene with HBr in presence of peroxide proceeds thro...
Text Solution
|
- When 3-phenylpropene reacts with HBr in the presence of peroxide, the ...
Text Solution
|
- The major product obtained by the addition reaction of HBr to 4-methyl...
Text Solution
|
- Observe the following reactions and predict the nature of A and B
Text Solution
|
- In presence of peroxide, hydrogen chloride and hydrogen iodide do not ...
Text Solution
|
- Acid catalysed hydration of alkene is an example for
Text Solution
|
- 2,3-Dimethyl-2-butene can be prepared by heating which of the followin...
Text Solution
|
- Acid catalysed hydration of alkenes except ethene leads to the formati...
Text Solution
|
- 2-Phenyl propene on acidic hydration gives ,
Text Solution
|
- Among the alkenes, which one produces tertiary butyl alcohol on acid h...
Text Solution
|