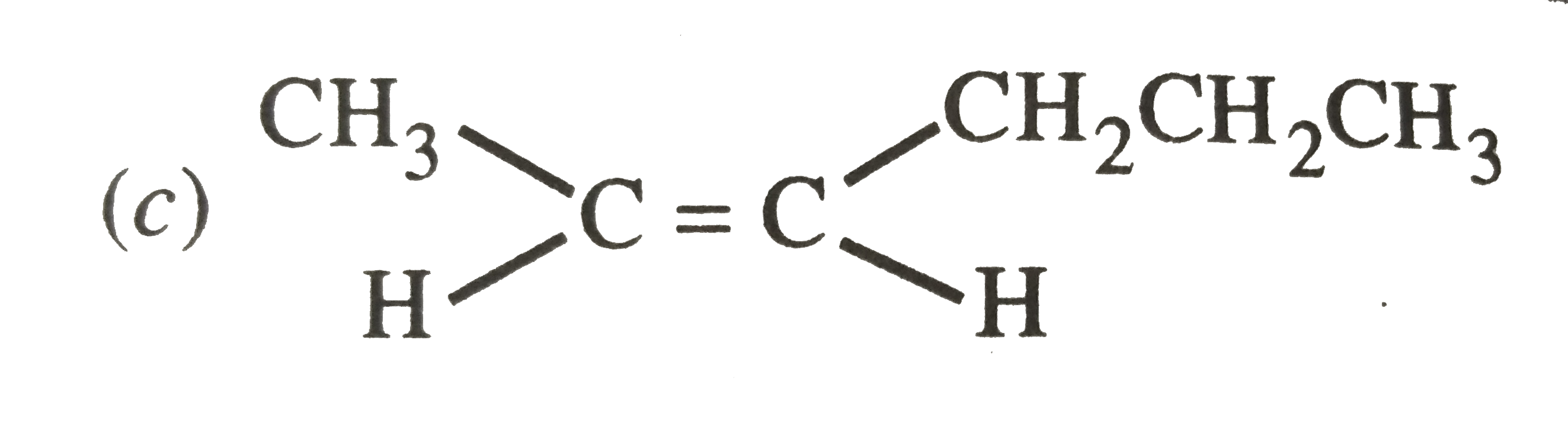
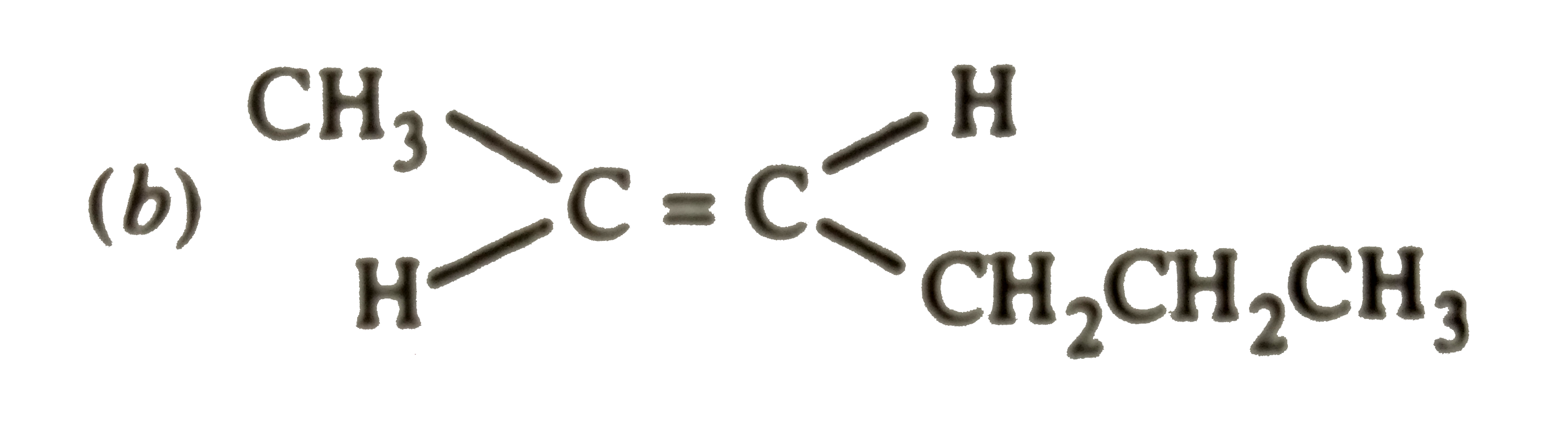A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
PRADEEP|Exercise Competition Focus (JEE(main and advanced)/Medical Entrance) II. MULTIPLE CHOICE|14 VideosHYDROCARBONS
PRADEEP|Exercise Competition Focus (JEE(main and advanced)/Medical Entrance) III. MULTIPLE CHOICE|14 VideosHYDROCARBONS
PRADEEP|Exercise ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS (PROBLEMS)|2 VideosEQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSES
PRADEEP|Exercise Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)|10 VideosHYDROGEN
PRADEEP|Exercise COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2|15 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-HYDROCARBONS-Competition Focus (JEE(main and advanced)/Medical Entrance) I. MULTIPLE CHOICE QUESTIONS
- 2-Hexyne gives trans -2- hexene on treatment with :
Text Solution
|
- The isomerism of 2-butyne to 1-butyne can be achieved by treatment wit...
Text Solution
|
- Identify X in the following sequence of reactions : CH3-undersetunde...
Text Solution
|
- Catalyst used in dimerisation of acetylene to prepare chloroprene is
Text Solution
|
- Which of the following reagents will be able to distinguish between 1-...
Text Solution
|
- Propyne and propene can be distinguished by :
Text Solution
|
- Ethylene can be separated from acetylene by passing the maxiture throu...
Text Solution
|
- 1-Butyne reacts with cold alkaline KMnO(4) to produce
Text Solution
|
- A compound X (C5H8) reacts with ammoniacal AgNO3 to give a white preci...
Text Solution
|
- CH3-C-=C-CH3 underset((ii)H2O //Zn)overset((i)X)to CH3-undersetunderse...
Text Solution
|
- CH-=CH overset(O3)to X overset(Zn//CH3OH)to Y, Y is
Text Solution
|
- Identify a reagent from the following list which can easily distinguis...
Text Solution
|
- The molecular formula of diphenylmethane , , is C(13)H(12), How m...
Text Solution
|
- The hydrocarbon which does not decolourise alkaline KMnO4 solution and...
Text Solution
|
- The correct statement is :
Text Solution
|
- Which one of the following is an aromatic compound ?
Text Solution
|
- Which of the following compounds is not aromatic ?
Text Solution
|
- From the following compounds choose the one which is not aromatic ?
Text Solution
|
- The chemical system that is non- aromatic is
Text Solution
|
- Among the following the aromatic compound is
Text Solution
|