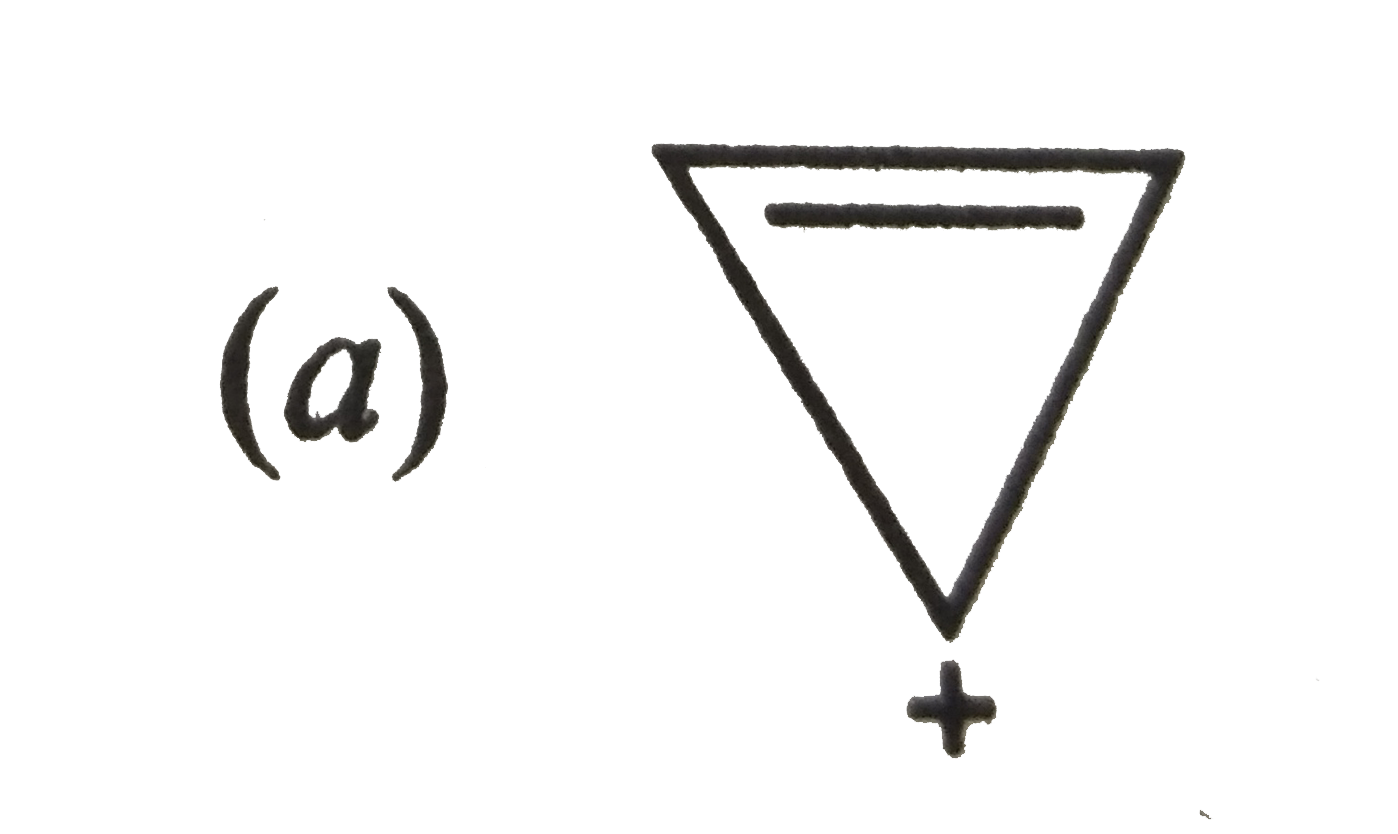
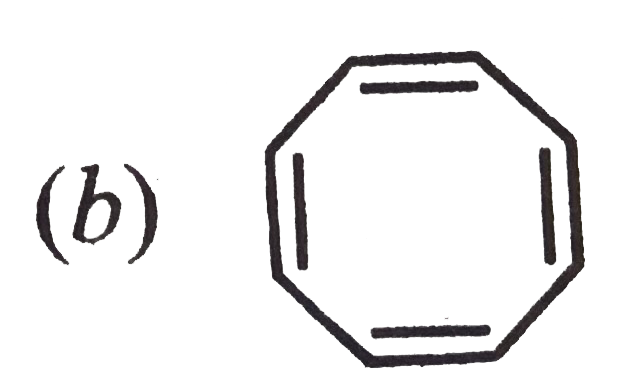
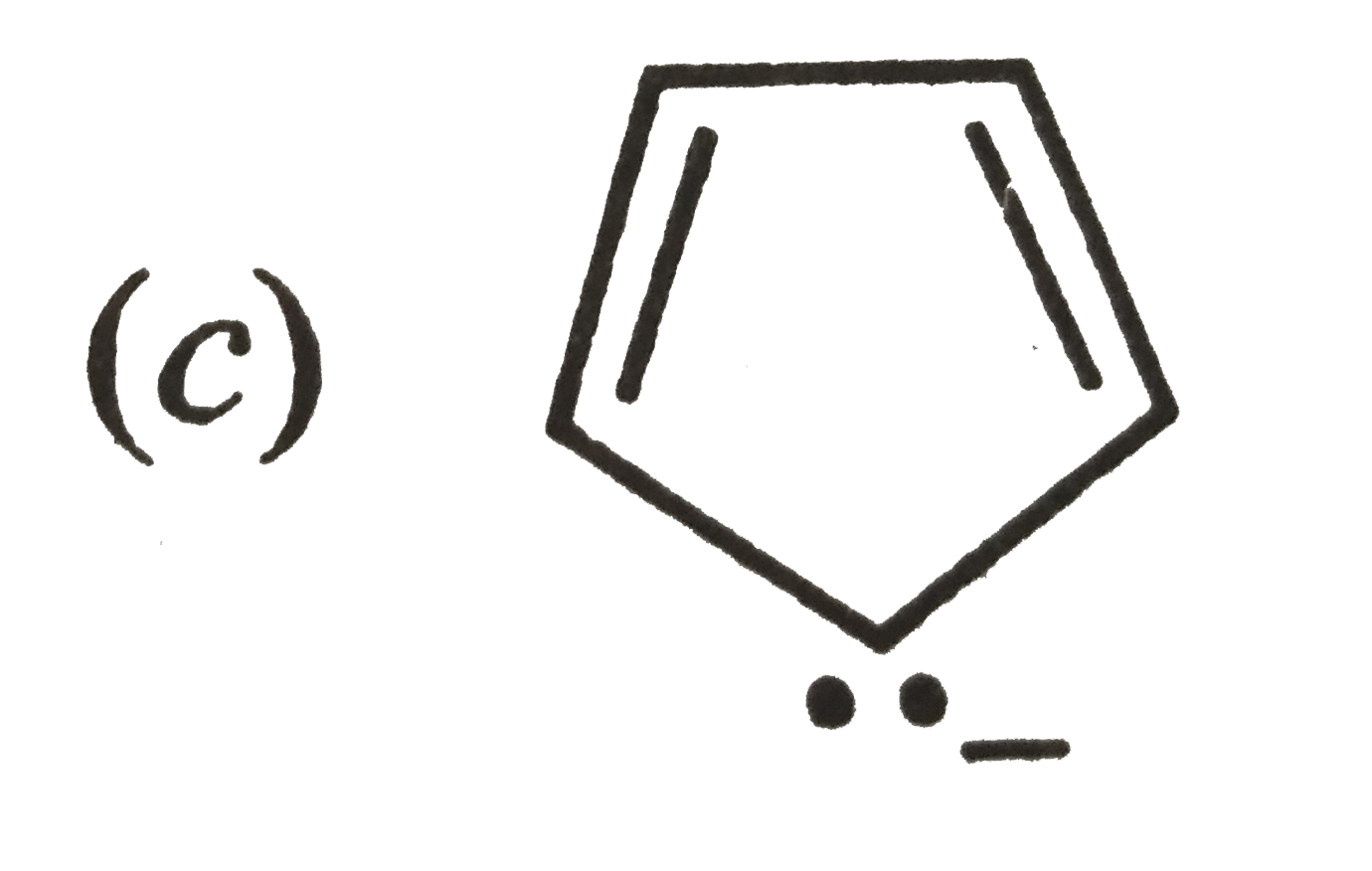
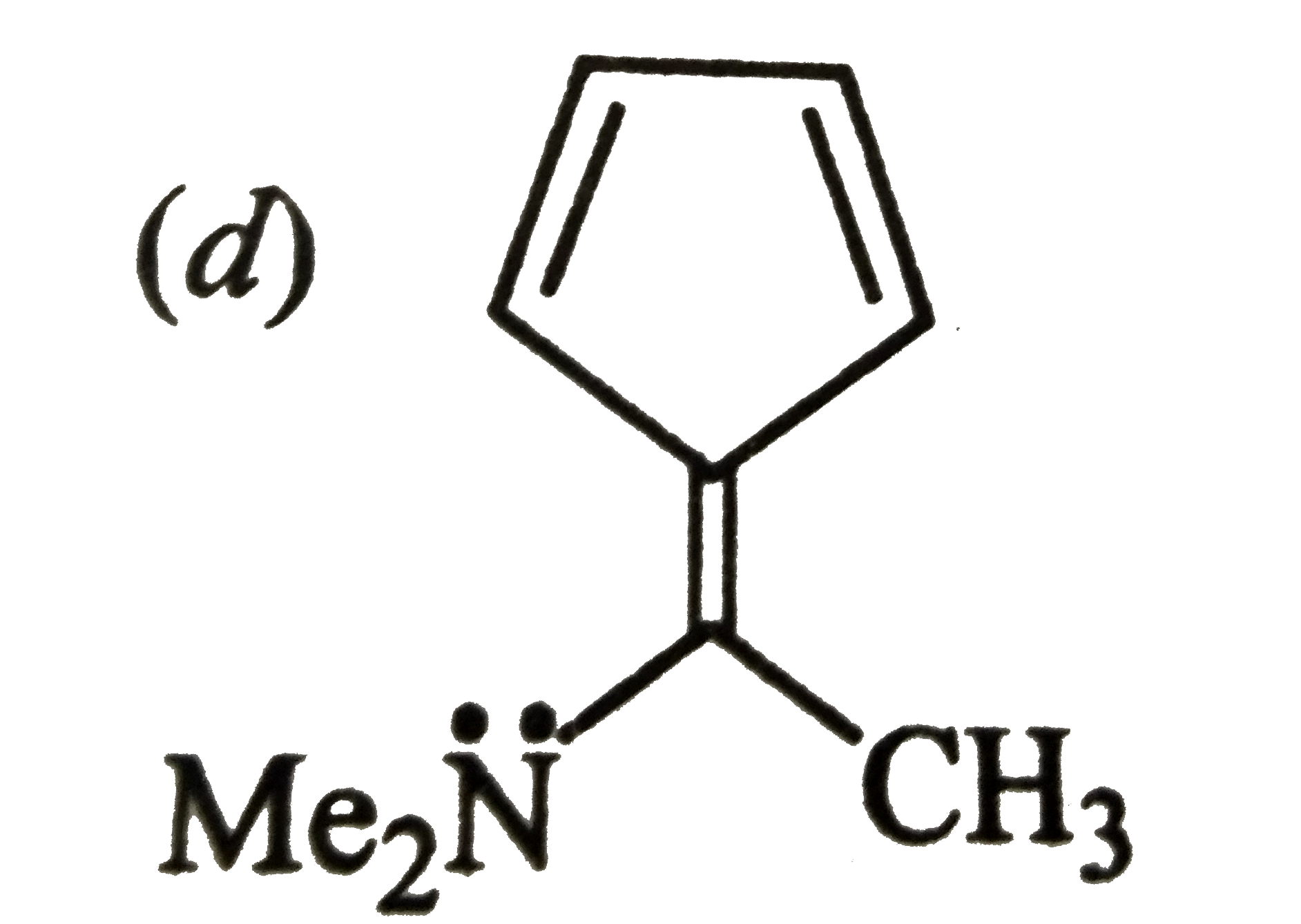
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
PRADEEP|Exercise Competition Focus (JEE(main and advanced)/Medical Entrance) II. MULTIPLE CHOICE|14 VideosHYDROCARBONS
PRADEEP|Exercise Competition Focus (JEE(main and advanced)/Medical Entrance) III. MULTIPLE CHOICE|14 VideosHYDROCARBONS
PRADEEP|Exercise ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS (PROBLEMS)|2 VideosEQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSES
PRADEEP|Exercise Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)|10 VideosHYDROGEN
PRADEEP|Exercise COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2|15 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-HYDROCARBONS-Competition Focus (JEE(main and advanced)/Medical Entrance) I. MULTIPLE CHOICE QUESTIONS
- Which one of the following is an aromatic compound ?
Text Solution
|
- Which of the following compounds is not aromatic ?
Text Solution
|
- From the following compounds choose the one which is not aromatic ?
Text Solution
|
- The chemical system that is non- aromatic is
Text Solution
|
- Among the following the aromatic compound is
Text Solution
|
- Which of the following compounds are aromatic ?
Text Solution
|
- Which is a non-aromatic compound
Text Solution
|
- The non aromatic compound among the following is -
Text Solution
|
- The radical is aromatic because it has
Text Solution
|
- Which of the following is not aromatic
Text Solution
|
- Cycloheptatrienyl cation is :
Text Solution
|
- The most likely protonation site in the following molecule is
Text Solution
|
- Which one of the following is an aromatic compound ?
Text Solution
|
- The total number of aromatic species genetic the following reactions i...
Text Solution
|
- Given : The enthalpy of hydrogenation of these compounds will be...
Text Solution
|
- In reaction C6H5CH3overset"oxidation"to A overset"NaOH"to B overset"...
Text Solution
|
- What is the electrophile when RCl + AlCl3 are used in Friedel-Crafts r...
Text Solution
|
- In the given reaction, the product P is
Text Solution
|
- The treatment of benzene with isobutene in the presence of sulphuric a...
Text Solution
|
- When Friedel-Crafts alkylation of benzene is carried out with n-propyl...
Text Solution
|