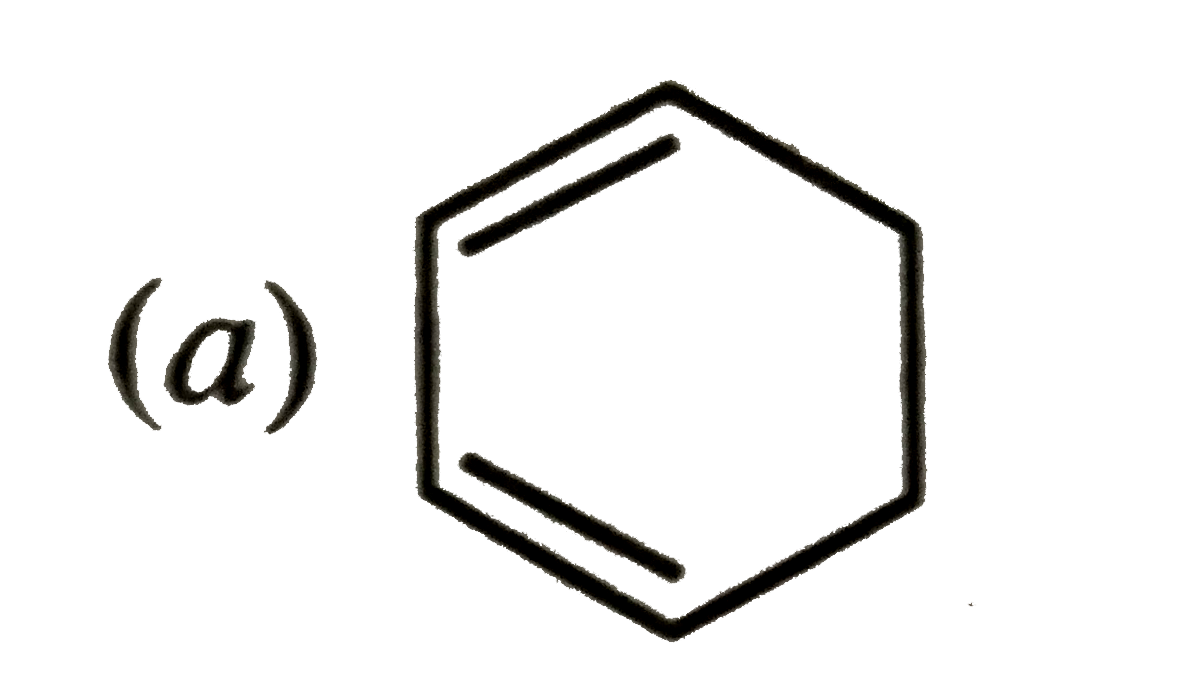
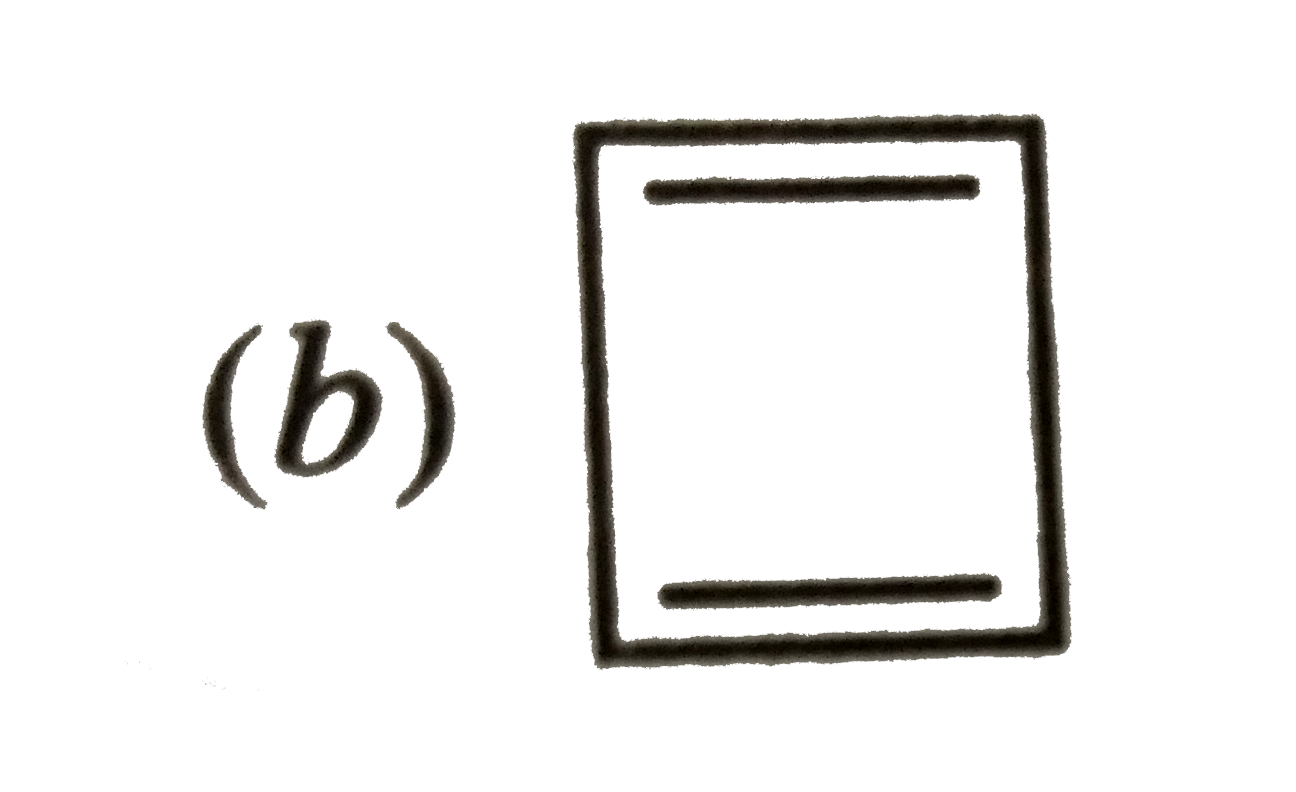
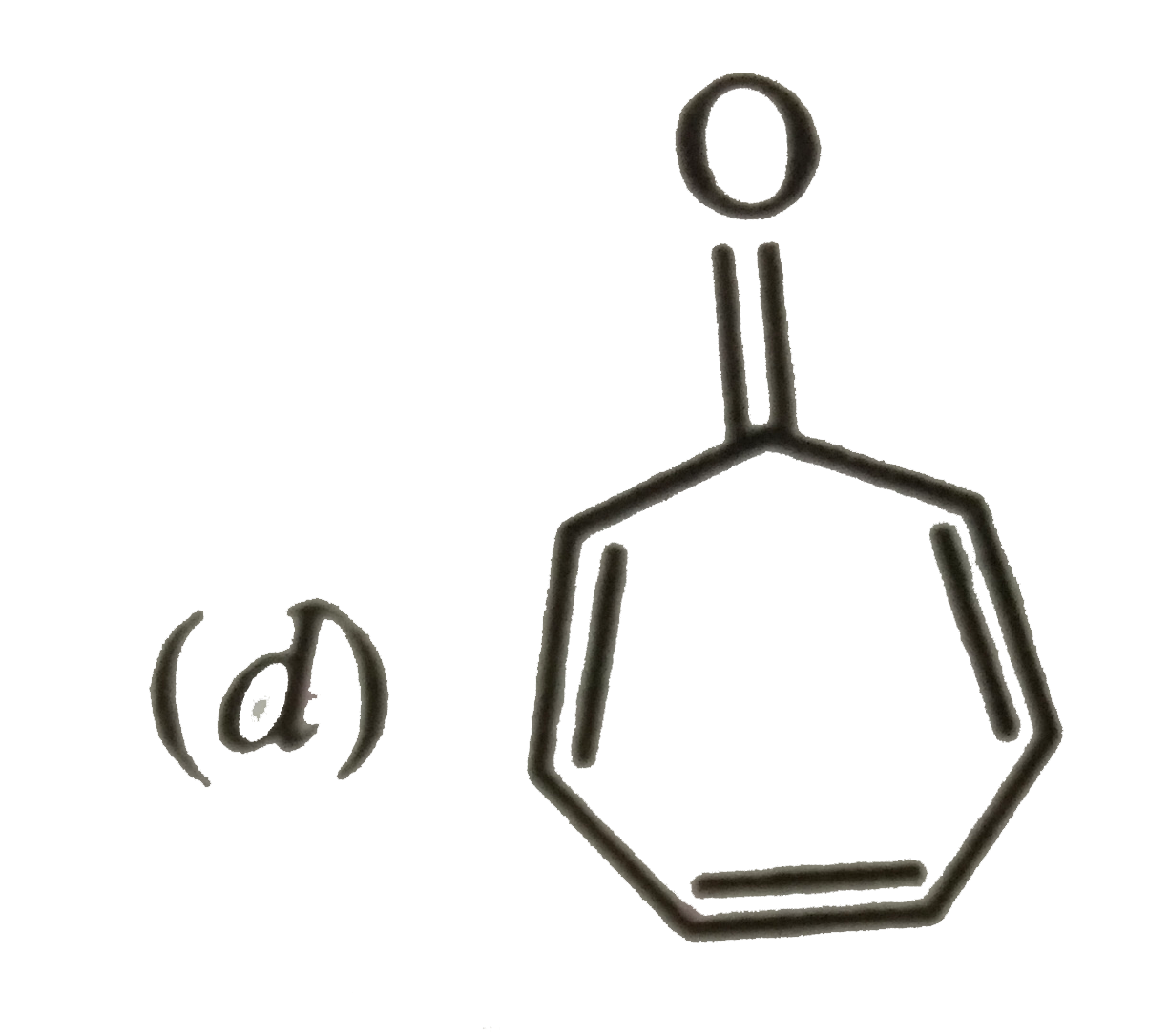
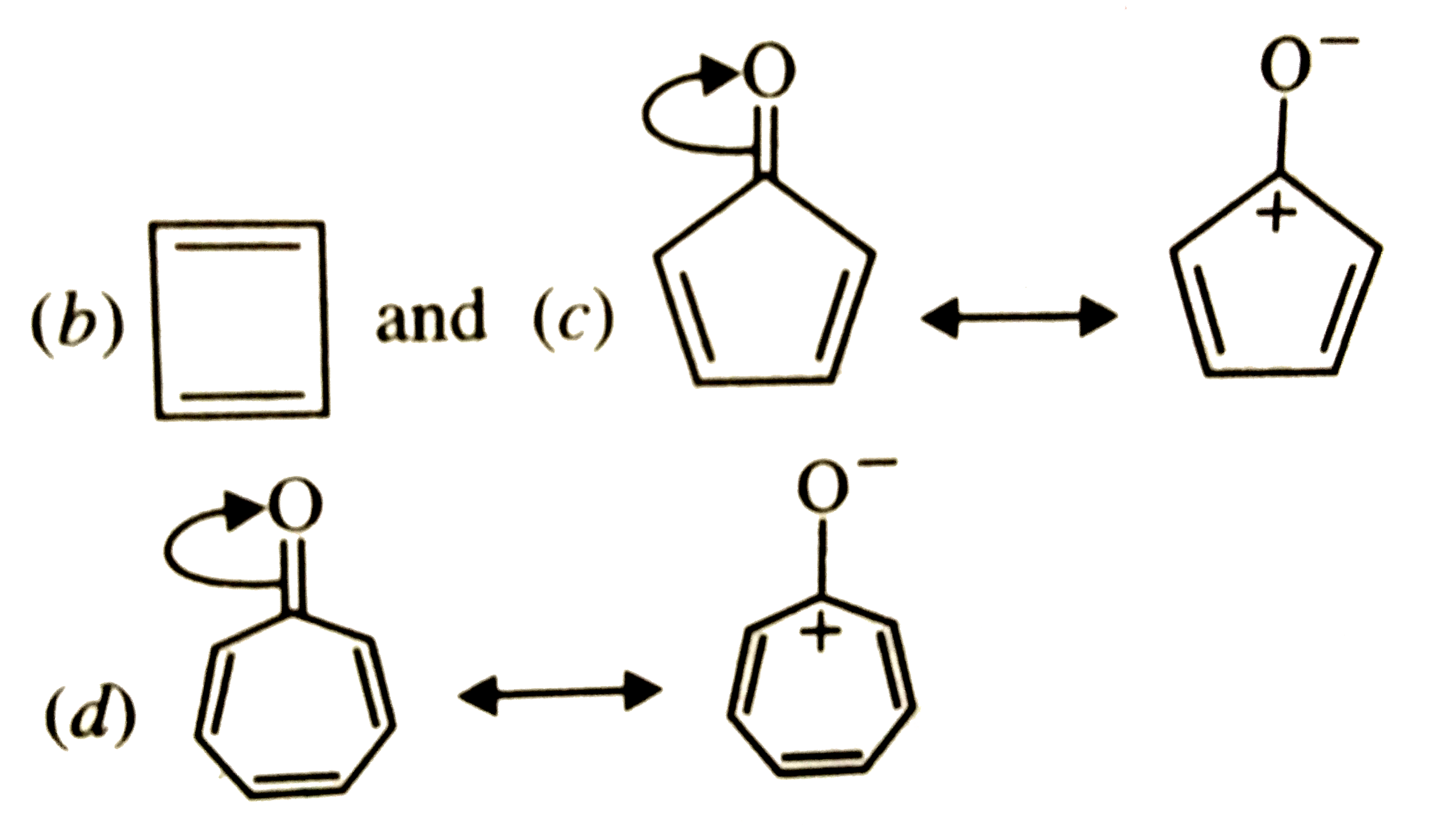
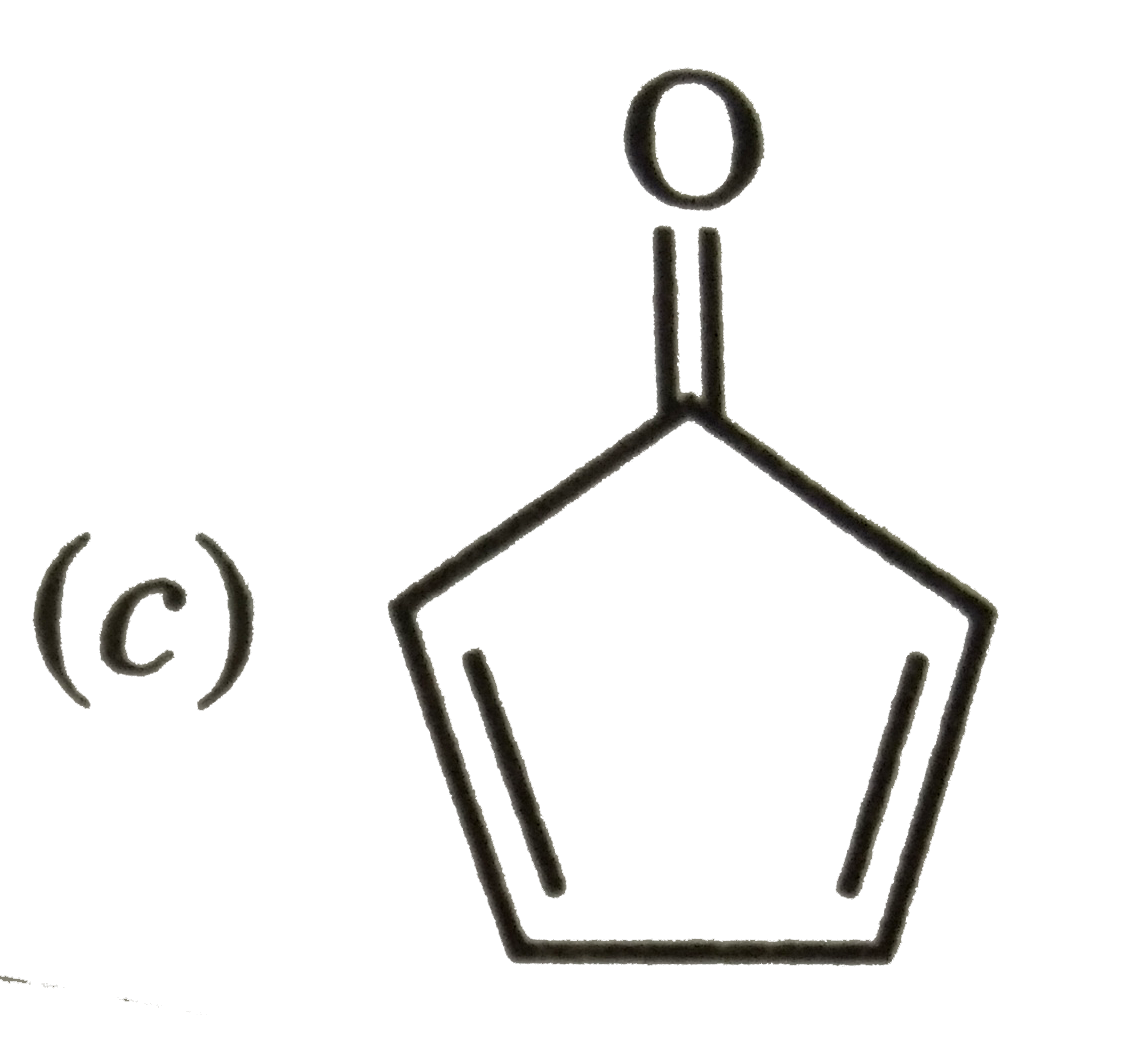A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
PRADEEP|Exercise Competition Focus (JEE(main and advanced)/Medical Entrance) III. MULTIPLE CHOICE|14 VideosHYDROCARBONS
PRADEEP|Exercise Competition Focus (JEE(main and advanced)/Medical Entrance) IV. MATCHING TYPE QUESTIONS|3 VideosHYDROCARBONS
PRADEEP|Exercise Competition Focus (JEE(main and advanced)/Medical Entrance) I. MULTIPLE CHOICE QUESTIONS|189 VideosEQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSES
PRADEEP|Exercise Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)|10 VideosHYDROGEN
PRADEEP|Exercise COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2|15 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-HYDROCARBONS-Competition Focus (JEE(main and advanced)/Medical Entrance) II. MULTIPLE CHOICE
- In the Newman projection for 2,2-dimethylbutane X and Y can respe...
Text Solution
|
- Which of the following compounds have finite dipole moments ?
Text Solution
|
- The correct statement concerning the structures E,F and G is(are )
Text Solution
|
- Which of the following alkenes on treatment with HBr in presence or ab...
Text Solution
|
- Dehydration of which one of the following alcohols produces an alkene ...
Text Solution
|
- An unsaturated hydrocarbon on ozonolysis gives one mole each of methan...
Text Solution
|
- Among the following reactions (s), which gives (give) tert-butyl benze...
Text Solution
|
- Which of the following on treatment with warm dil. H2SO4 in presence o...
Text Solution
|
- Amongst the given option, the compound(s) in which all the atoms are i...
Text Solution
|
- Which of the following molecules, in pure form, is /are ustable at roo...
Text Solution
|
- Among P,Q ,R and S, the aromatic compound is (are )
Text Solution
|
- Which of the following compounds on decarboxylation with soda-lime wil...
Text Solution
|
- Which of the following undergoes electrophilic substitution reactions ...
Text Solution
|
- Which of the folllowing on reductive ozonolysis will give only glyoxal...
Text Solution
|