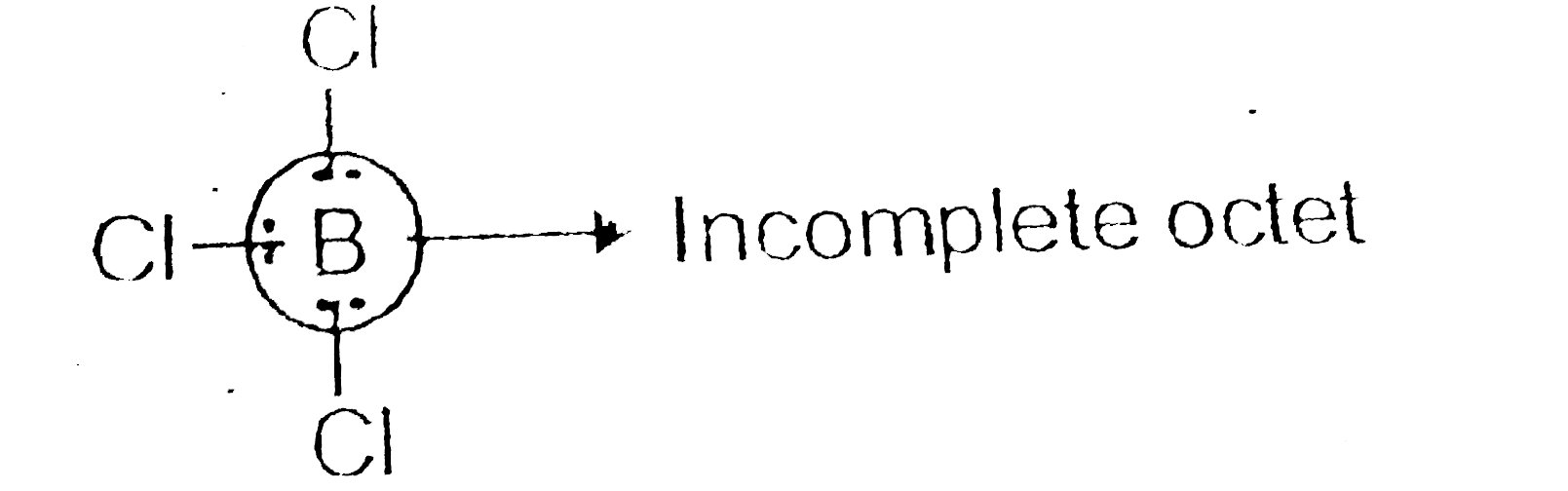Text Solution
Verified by Experts
Topper's Solved these Questions
EQUILIBRIUM
AAKASH INSTITUTE|Exercise Assignment (SECTION-A) (SUBJECTIVE TYPE QUESTIONS(ONE OPTION IS CORRECT)|45 VideosEQUILIBRIUM
AAKASH INSTITUTE|Exercise Assignment (SECTION-B)(OBJECTIVE TYPE QUESTIONS (ONE OPTION IS CORRECT)|20 VideosEQUILIBRIUM
AAKASH INSTITUTE|Exercise ASSIGNMENT (SECTION -D)|20 VideosENVIRONMENTAL CHEMISTRY
AAKASH INSTITUTE|Exercise ASSIGNMENT (SECTION-D) (Assertion - Reason Type Questions)|5 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
AAKASH INSTITUTE|Exercise Assignment (Section-D) Assertion-Reason Type Question|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-EQUILIBRIUM-TRY YOURSELF
- Mention atleast three ways by which the concentration of SO(2)(g) be i...
Text Solution
|
- Name a species which can act both as conjugate acid and conjugate base...
Text Solution
|
- BCl(3) behavs a a Lewis acid Give reason.
Text Solution
|
- Calculte the pof (N)/(1000) sodium hydroxide (NaOH) solution assuming ...
Text Solution
|
- 13.5 g of an acid HA of molecular mass 135 was dissolved in 10 litres ...
Text Solution
|
- Calculate t the H^(+) ion concentration in 0.10 M acetic acid solution...
Text Solution
|
- When 0.1 mole of NH(3) is dissolved in water to make 1.0 L of solution...
Text Solution
|
- If ionisation constant of an acid (HA) at equlibrium is 1.0xx10^(-8) t...
Text Solution
|
- Calcuate the degree of ionisation and pH of 0.05 M solution of a weak ...
Text Solution
|
- The ionization constnt of (C(2)H(5))(3)N is 6.4xx10^(-5). Calculate it...
Text Solution
|
- Calculate the pH of 0.033 M ammonia solution if 0.033 M NH(4)Cl is int...
Text Solution
|
- Calculate the pH of 0.01 M solution of NH(4)CN. The dissociation const...
Text Solution
|
- One litre of 0.05 M HCl was completely neutralized by NaOH. Calcuate t...
Text Solution
|
- The solubilty of barium sulphate at 298 K is 1.1 xx 10^(-5) "mol " L^(...
Text Solution
|
- 20 mL of 1.5 xx 10^(-5) M barium chloride solution is mixed with 40 mL...
Text Solution
|
- Calculate solubility product of Hg(2)Cl(2) if its solubility at room t...
Text Solution
|
- NaCl solution is added to a saturated solution of PbCl(2). What will h...
Text Solution
|
- Given the mathematical expression for the equilibrium constant Kc for...
Text Solution
|
- The following concentration were obtained for the formation of NH3 fro...
Text Solution
|
- The value of the equilibrium constant for the reaction H2(g) +I2(g) ...
Text Solution
|