A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS
AAKASH INSTITUTE|Exercise ASSIGMENT (SECTION-C )|11 VideosSOLUTIONS
AAKASH INSTITUTE|Exercise ASSIGMENT (SECTION-D ) LINKED COMPRESHENSION TYPE QUESTIONS|6 VideosSOLUTIONS
AAKASH INSTITUTE|Exercise ASSIGMENT (SECTION-A)|50 VideosREDOX REACTIONS
AAKASH INSTITUTE|Exercise ASSIGNMENT SECTION - D|10 VideosSOME BASIC CONCEPT OF CHEMISTRY
AAKASH INSTITUTE|Exercise ASSIGNMENT( SECTION - D) Assertion-Reason Type Questions|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-SOLUTIONS-ASSIGMENT (SECTION-B)
- If relative decrease in vapour pressure is 0.4 for a solution containi...
Text Solution
|
- Which of the following pair of solutions can be expected to be isotoni...
Text Solution
|
- 2.56 f of suphur in 100 g of CS(2) has depression in freezing point of...
Text Solution
|
- Acetic acid dimerises in benzene solution.The van't Hoff factor for th...
Text Solution
|
- When 20 g of napthanoic acid (C(11)H(8)O(2)) is dissolved in 50 g of b...
Text Solution
|
- A 0.2 molal aqueous solution of a weak acid HX is 20% ionized. The fre...
Text Solution
|
- If a solute undergoes dimerisation and trimerisation, the minimum valu...
Text Solution
|
- A water sample contains 9.5% MgCl(2) and 11.7% NaCl (by weight).Assumi...
Text Solution
|
- A complex is written as M(en)(y)Br. Its 0.05 molar solution shows 2.46...
Text Solution
|
- 20 g of non-electrolyte, non-volatile solute (C(x)H(2x)O(6)) when diss...
Text Solution
|
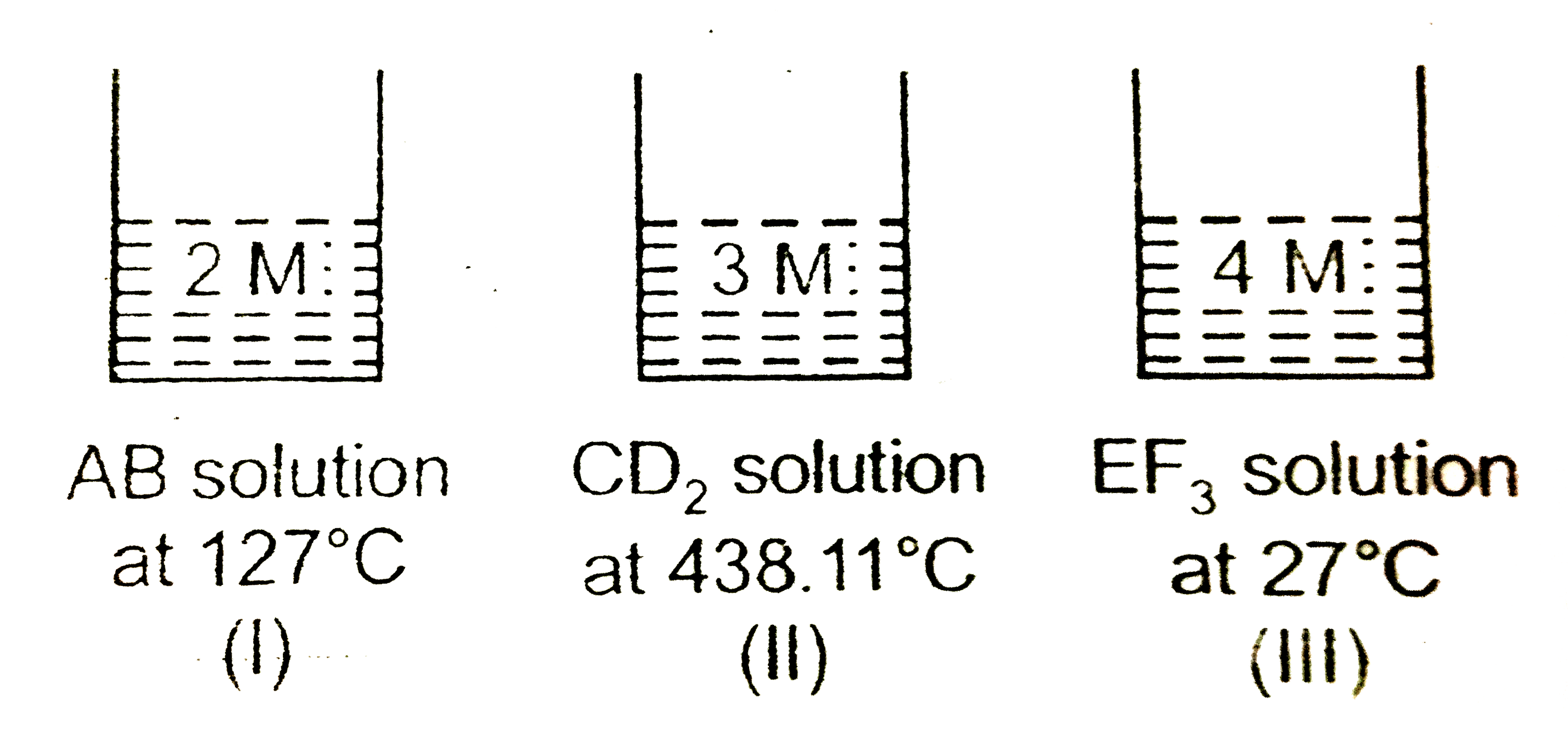
- Consider threee solutions of 3 strong electrolytes.AB, CD(2) and EF(3)...
Text Solution
|
- At 27^(@)C,3.92 gm H(2)SO(4) is present in 250 ml solution. The osmoti...
Text Solution
|
- Degree of dissociation of three binary electrolytes AB, CD and EF are...
Text Solution
|
- 0.067 molar aqueous solution of a binary electrolyte A^(+)B^(-) shows ...
Text Solution
|
- A' gram of non-volatile, non-electrolyte (molar mass M) is dissolved i...
Text Solution
|
- The vapour pressure of a solvent decreases by 5.4 torr when a non-vola...
Text Solution
|
- 75 g ethylene glycol is dissolved in 500 gram water. The solution is p...
Text Solution
|
- When mercuric iodide is added to aqueous KI solution:
Text Solution
|
- When a saturated solution of sodium chloride is heated it
Text Solution
|
- The solubility of a gas in a liquid generally generally increases with
Text Solution
|