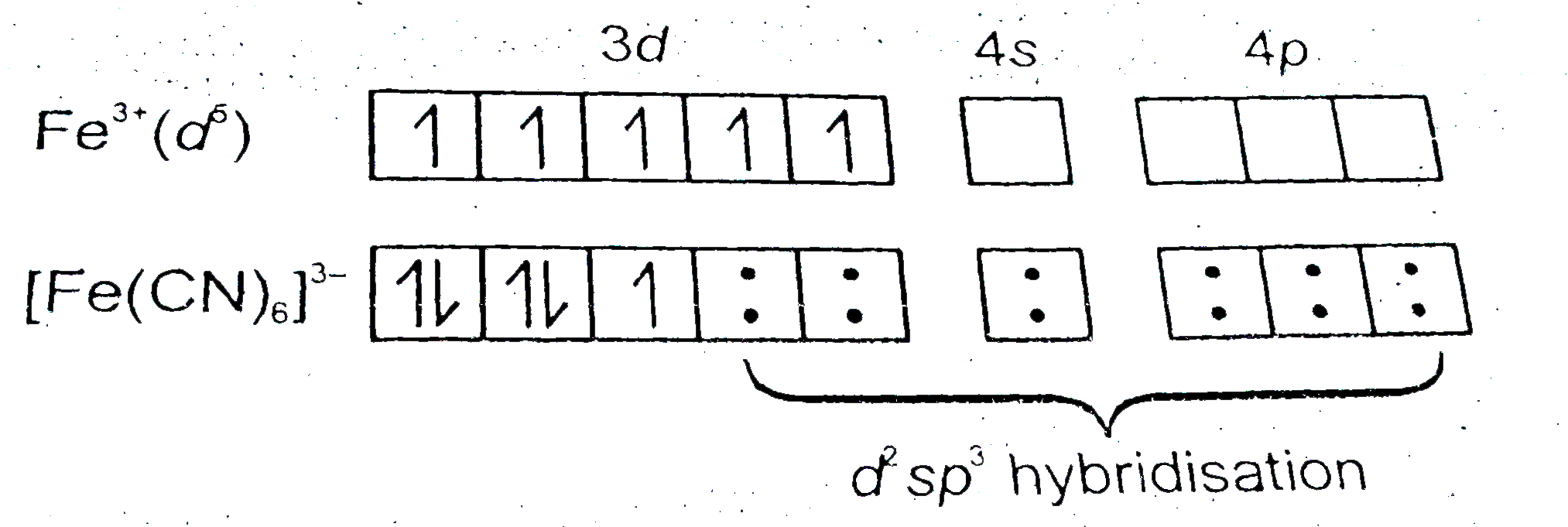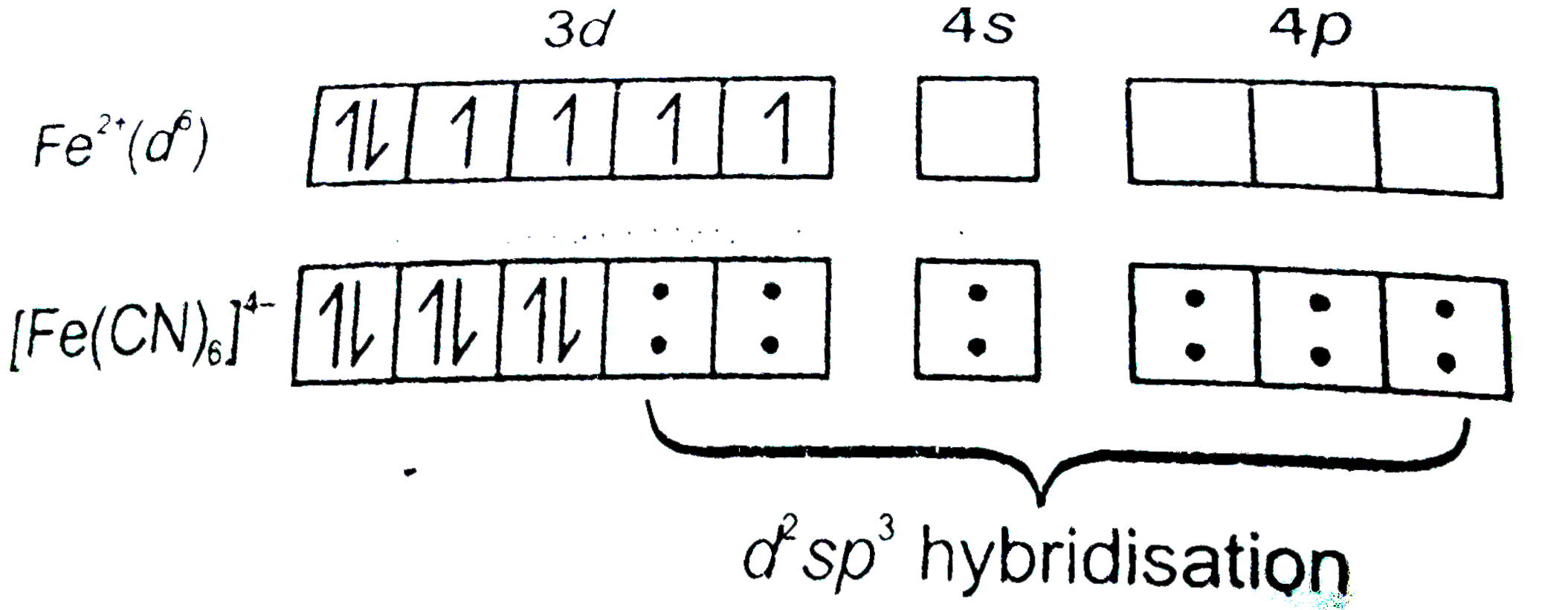Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
AAKASH INSTITUTE|Exercise Try Yourself|14 VideosCOORDINATION COMPOUNDS
AAKASH INSTITUTE|Exercise Assignment (Section-A Objective type Questions (One Option is correct))|45 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
AAKASH INSTITUTE|Exercise Assignment (Section - J Aakash Challengers Questions)|6 VideosELECTROCHEMISTRY
AAKASH INSTITUTE|Exercise Assignment (SECTION - J) (Aakash Challengers Questions)|10 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-COORDINATION COMPOUNDS -Assignment (Section-J Aakash Challenger Questions )
- How would you account for the magnetic behaviour of [Fe(CN)(6)]^(3-) a...
Text Solution
|
- For Ag^(+) metal ion, correct sequence regarding ligand strength is
Text Solution
|
- Choose the correct statement regarding complex CuF(6)^(-4)
Text Solution
|
- Which of the following ligands may be flexidentate ?
Text Solution
|
- In which complex C-O bond length is maximum ?
Text Solution
|
- Choose the incorrect regarding stability (a) [Fe("phen")(3)]^(+2) g...
Text Solution
|
- Choose the correct statement regarding complex CuF(6)^(-4)
Text Solution
|
- Strongest C-O bond is present in
Text Solution
|
- (MA(2)B(2)C(2))^(+n) can exhibit
Text Solution
|
- The hybridisation of Co in (CoF(6))^(-2) is
Text Solution
|
- Which of the following compound may be optical active ?
Text Solution
|