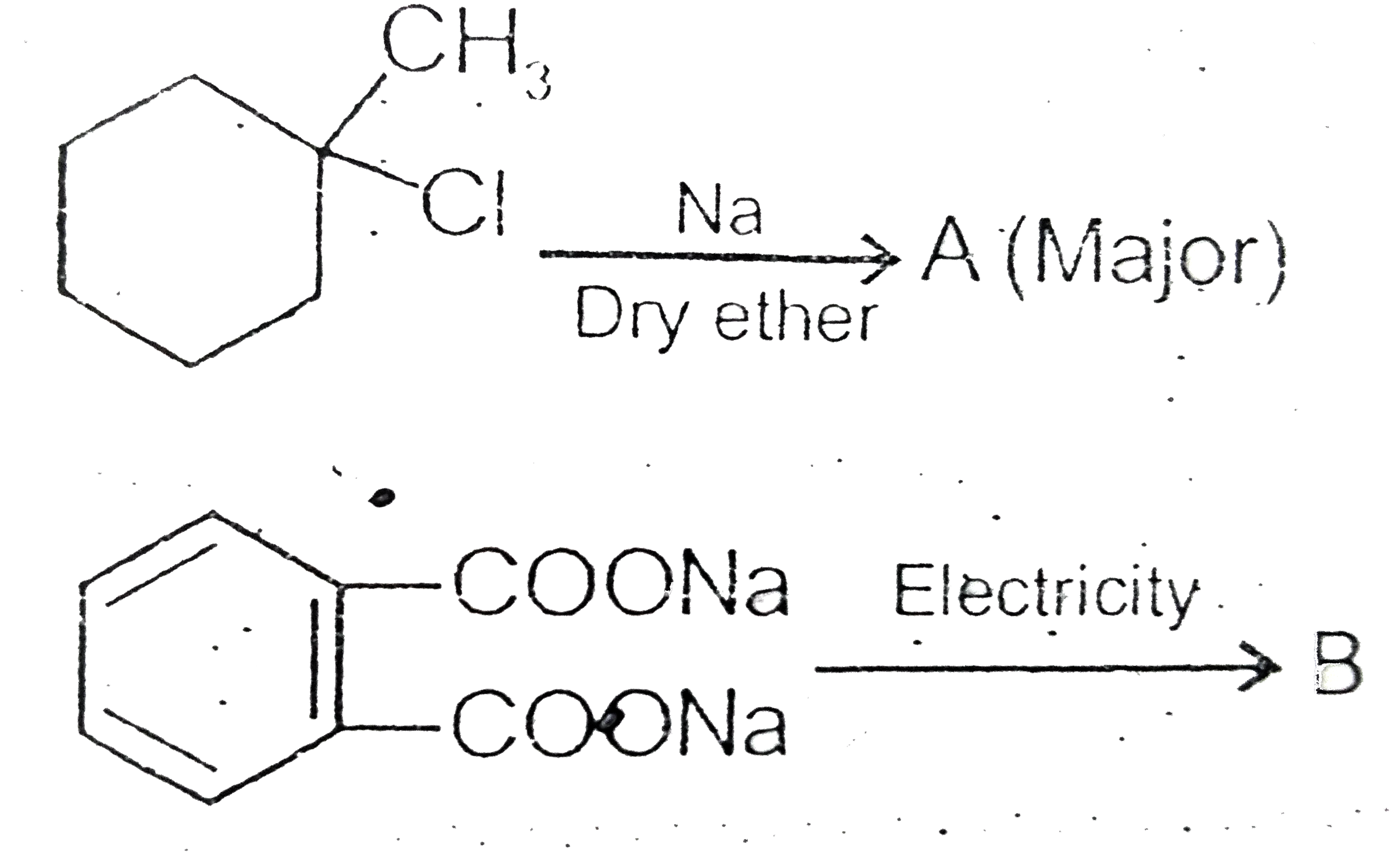
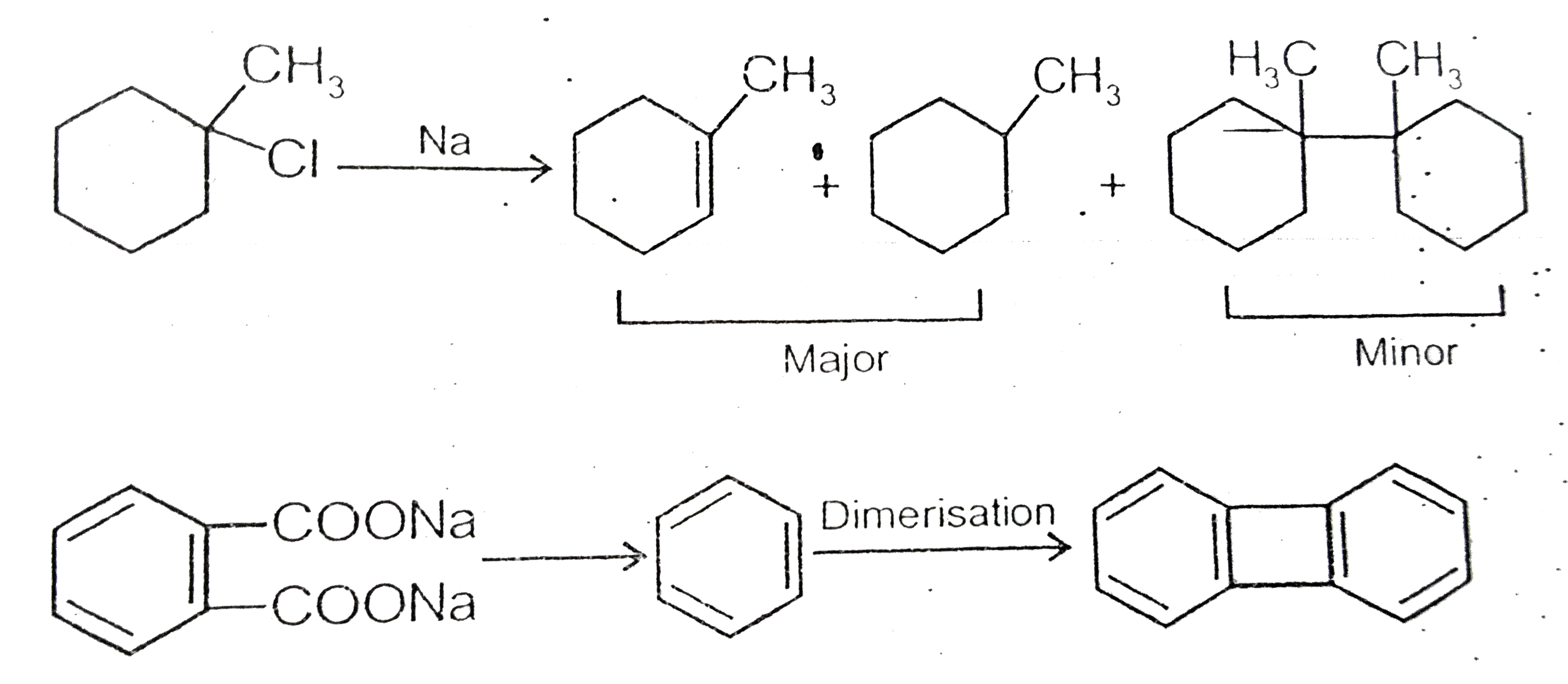
Text Solution
Verified by Experts
Topper's Solved these Questions
HYDROCARBONS
AAKASH INSTITUTE|Exercise ASSIGNMENT SECTION-A COMPETITION LEVEL DIFFER BY|50 VideosHYDROCARBONS
AAKASH INSTITUTE|Exercise SECTION-B OBJECTIVE TYPE QUESTIONS (ONE OPTION IS CORRECT)|25 VideosHYDROCARBONS
AAKASH INSTITUTE|Exercise Assignment(Section - D) (Assertion -Reason Type Question)|24 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE|Exercise ASSIGNMENT SECTION -D|15 VideosHYDROGEN
AAKASH INSTITUTE|Exercise ASSIGNMENT (SECTION - D) (Assertion-Reason Type question)|15 Videos
Similar Questions
Explore conceptually related problems