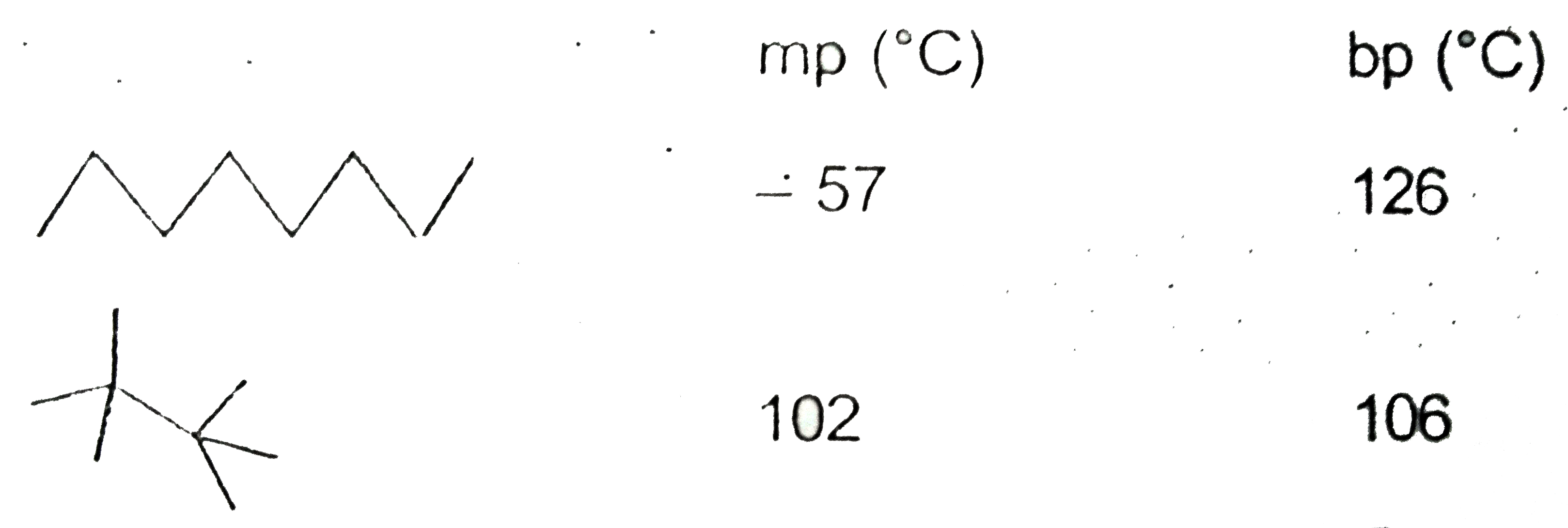Text Solution
Verified by Experts
Topper's Solved these Questions
HYDROCARBONS
AAKASH INSTITUTE|Exercise SECTION-J AAKASH CHALLENGERS QUESTIONS|5 VideosHYDROCARBONS
AAKASH INSTITUTE|Exercise TRY YOURSELF|78 VideosHYDROCARBONS
AAKASH INSTITUTE|Exercise SECTION-H MULTIPLE TRUE-FALSE TYPE QUESTIONS|2 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE|Exercise ASSIGNMENT SECTION -D|15 VideosHYDROGEN
AAKASH INSTITUTE|Exercise ASSIGNMENT (SECTION - D) (Assertion-Reason Type question)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-HYDROCARBONS-SECTION-I SUBJECTIVE QUESTIONS
- During pyrolysis of alkanesC-C bonds break rather than C-H bonds Why?
Text Solution
|
- Propane is brominated in presence of UV light. All the isomeric produc...
Text Solution
|
- Phenyl subsituted hydrocarbon (A) molecular mass 120 on monobrominatio...
Text Solution
|
- Write all the products obtained by treatment of n-hexane with diazome...
Text Solution
|
- Three compounds A,B and C all have molecular formula C(6)H(8) All the ...
Text Solution
|
- An organic compound (A) of molecular formula C(5)H(8) when treated wit...
Text Solution
|
- A certain compound 'A' has a molecular formula C(5)H(11)Br. It reacts ...
Text Solution
|
- The hydrocarbon [A] adds one mole of hydrogen in the presence of a pla...
Text Solution
|
- The melting points and boiling points for two C(8)H(18 ) isomers are g...
Text Solution
|