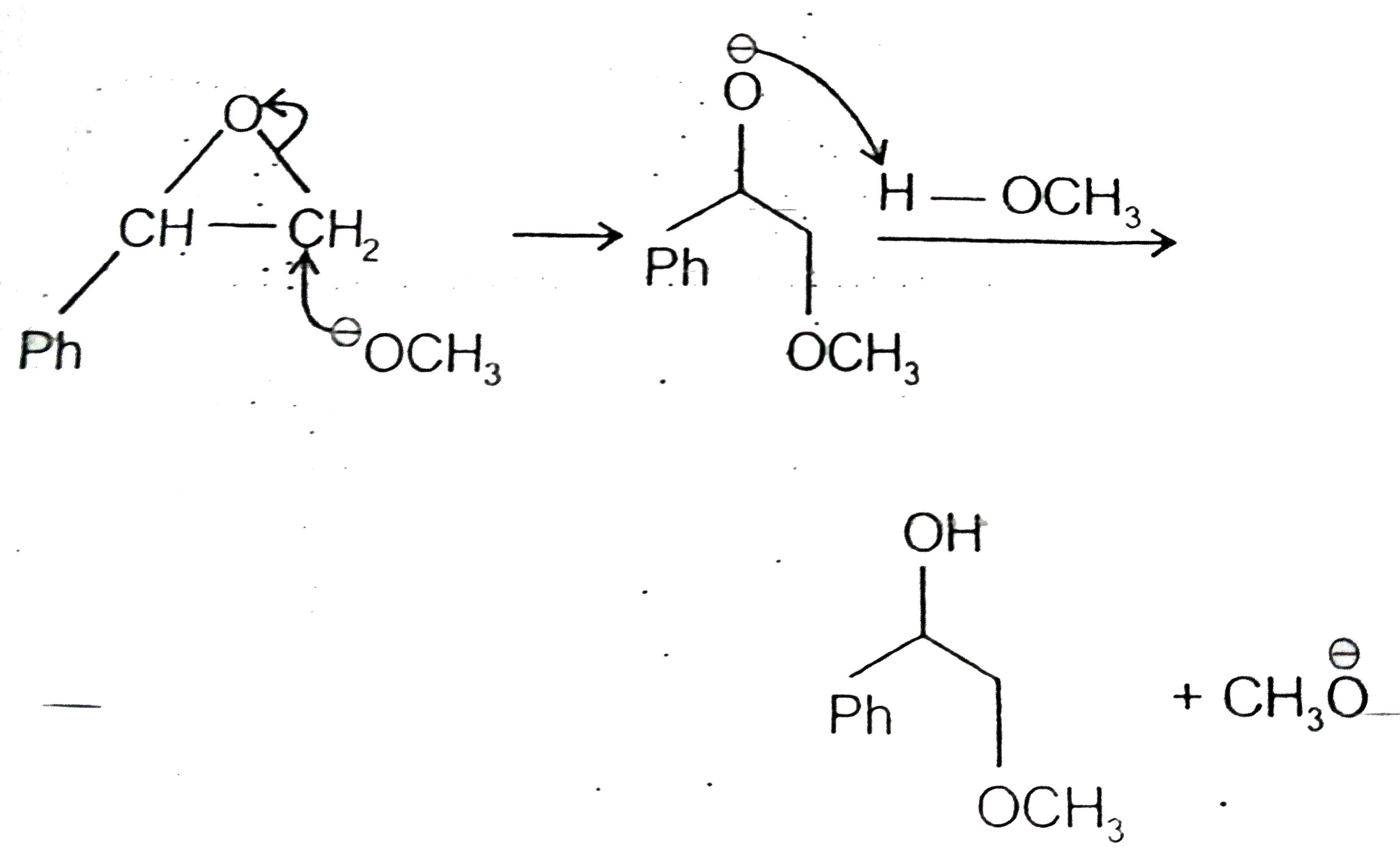
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Attack by a strong nucleophile such as CH(3)O^(Theta) (Methoxide i...
Text Solution
|
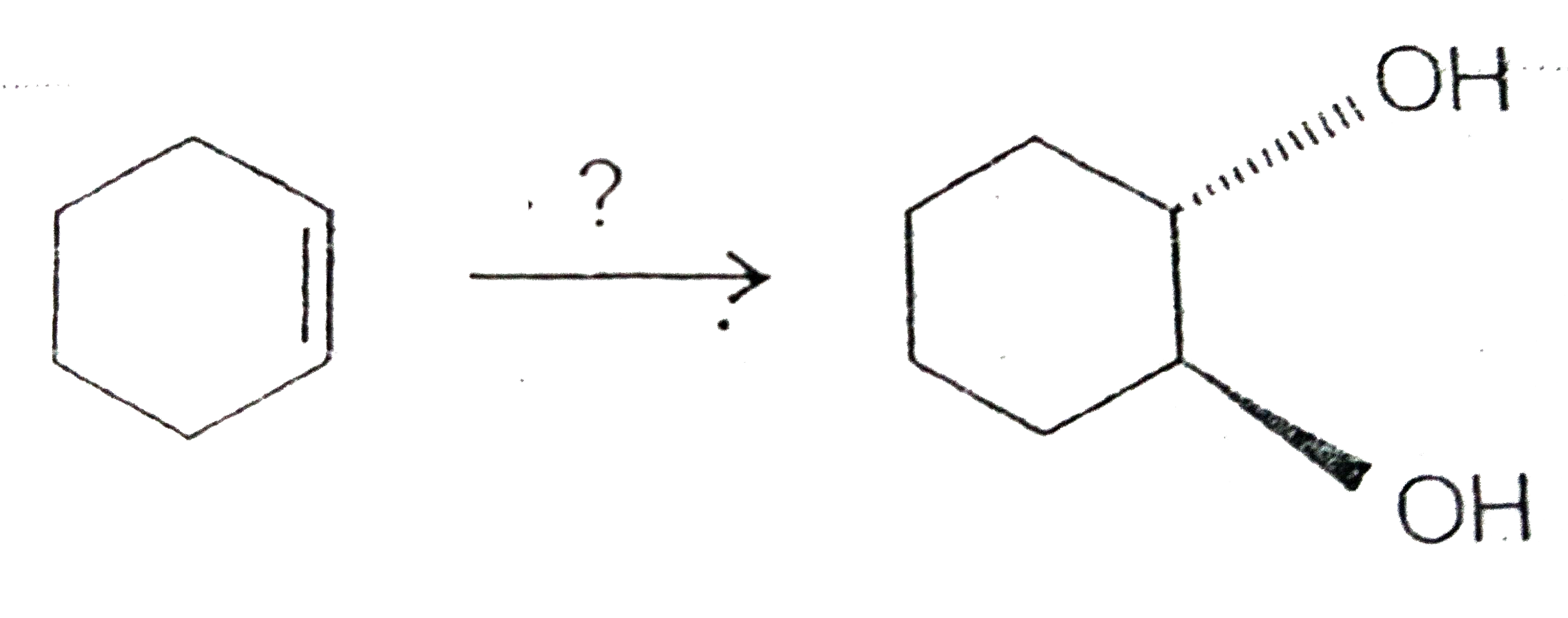
- Ring-opening reactions of epoxides can proceed by either S(N)2 or S(N)...
Text Solution
|
- Symmetrically subsituted epoxides give the same products in both the a...
Text Solution
|
- Symmetrically subsituted epoxides give the same products in both the a...
Text Solution
|
- Symmetrically subsituted epoxides give the same products in both the a...
Text Solution
|
- Symmetrically subsituted epoxides give the same products in both the a...
Text Solution
|
- Symmetrically subsituted epoxides give the same products in both the a...
Text Solution
|
- Aldehydes and ketones are amphoteric. Thus they can act both as acids ...
Text Solution
|
- Attack by a strong nucleophile such as CH(3)O^(Theta) (Methoxide ion )...
Text Solution
|