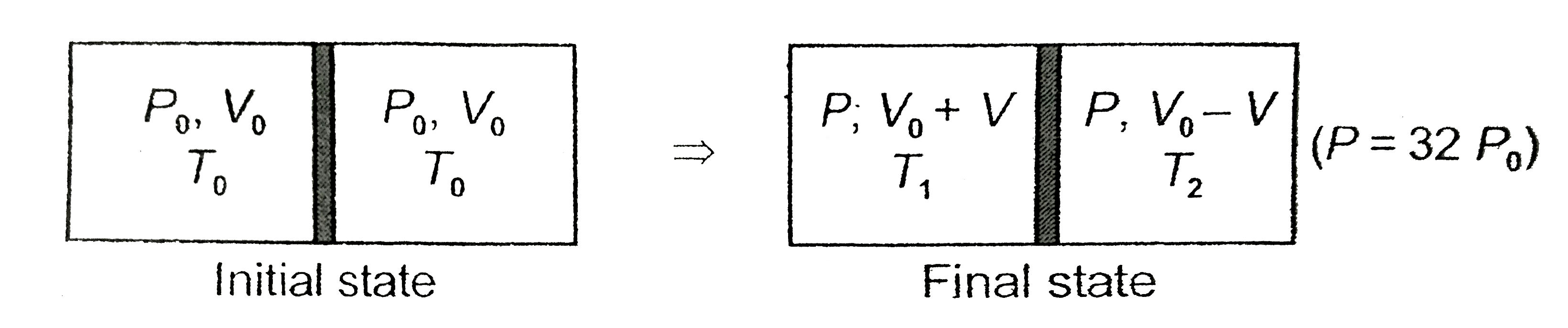Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
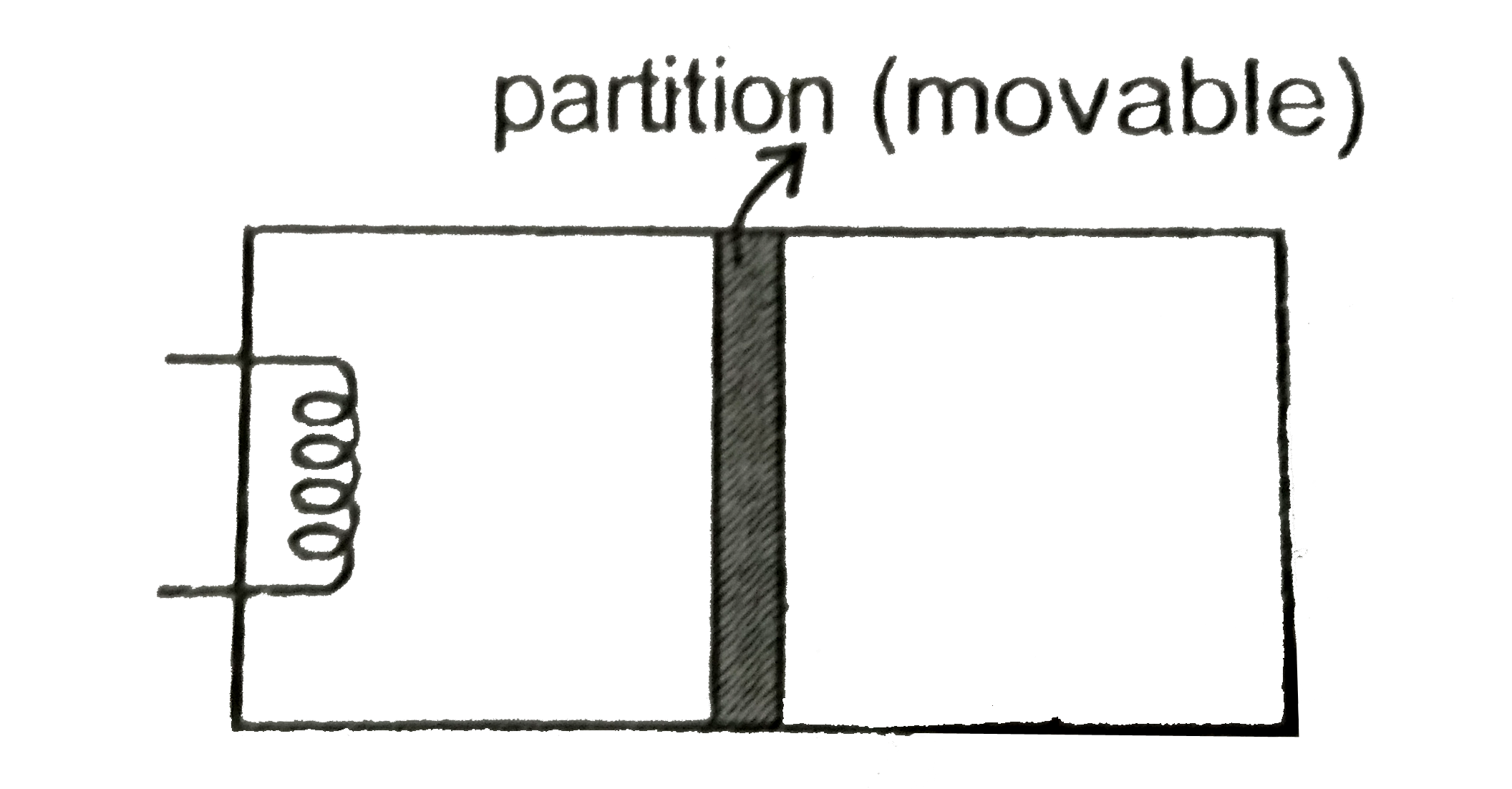
- A rectangualr box (shown in figure) has a movable and smooth portition...
Text Solution
|
- One mole of a monoatomic ideal gas occupies two champers of a cylinder...
Text Solution
|
- One mole of a monoatomic ideal gas occupies two champers of a cylinder...
Text Solution
|
- A rectangualr box (shown in figure) has a movable and smooth portition...
Text Solution
|
- An insulated container of gas has two chambers separated by an insulat...
Text Solution
|
- One mole of PCl(5) is heated in one litre closed container. If 0.6 mol...
Text Solution
|
- An insulated container of gas has two chambers separated by an insulat...
Text Solution
|
- The rectangular box shown in Fig has partition which can slide without...
Text Solution
|
- An insulated container of gas has two chambers separated by an insulat...
Text Solution
|