Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- An ideal gas undergoes a cyclic process as shown. Part of the process ...
Text Solution
|
- 100 moles of an ideal monatomic gas undergoes the thermodynamic proces...
Text Solution
|
- In a cyclic process shown in the figure an ideal gas is adial gas is a...
Text Solution
|
- During the adiabatic expansion of 2 moles of a gas, the internal energ...
Text Solution
|
- During an adiabatic expansion of 2 moles of a gas, the change in inter...
Text Solution
|
- The work done by 100 calorie of heat in isothermal expansion of ideal ...
Text Solution
|
- An ideal gas undergoes a cyclic process as shown. Part of the process ...
Text Solution
|
- In an isothermal process for an ideal gas
Text Solution
|
- During an adiabatic expansion of 2 moles of a gas, the change in inter...
Text Solution
|
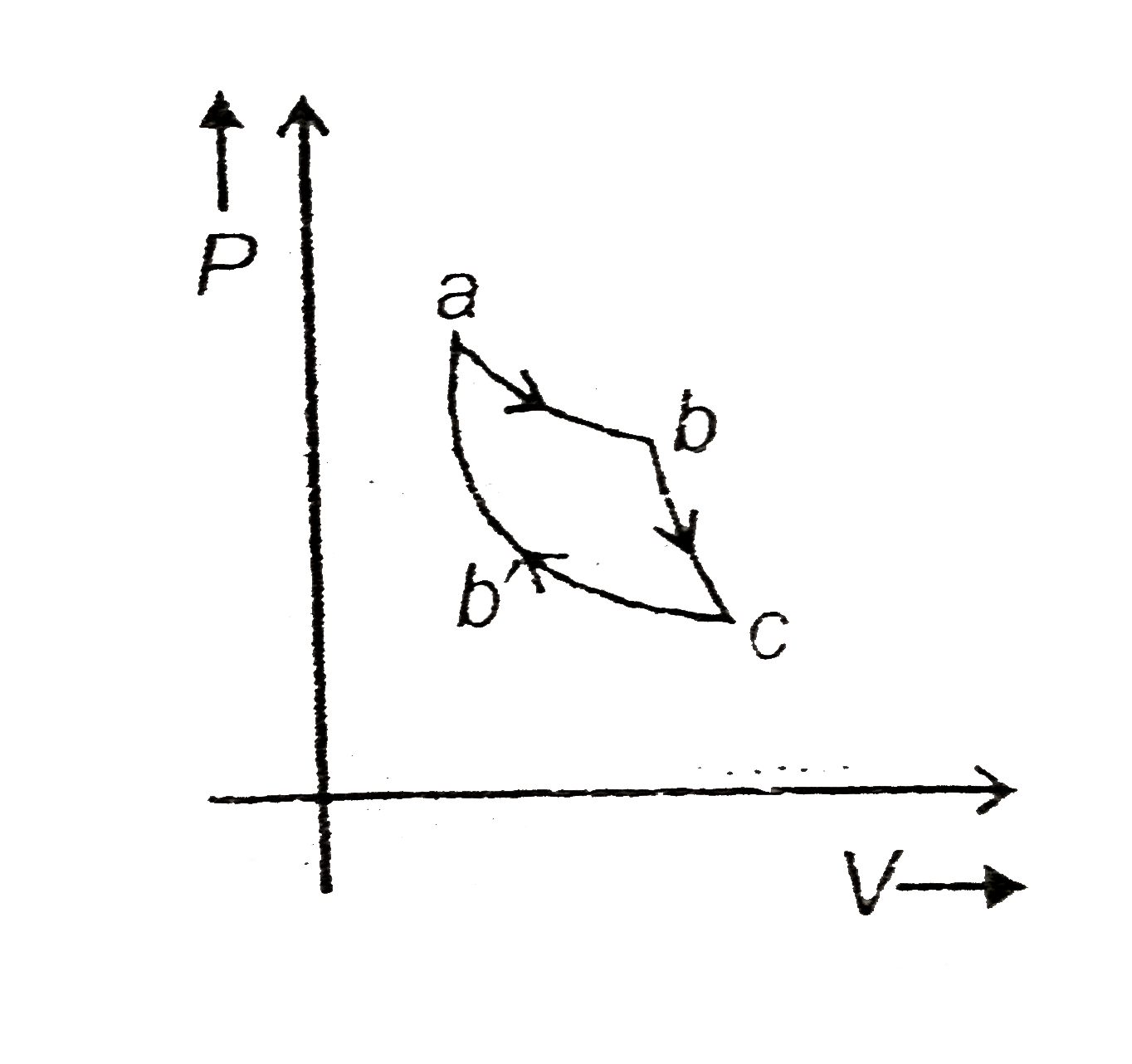 .
.