Similar Questions
Explore conceptually related problems
Recommended Questions
- Two benzyne intermediates are likely to be formed equally. Reaction wi...
Text Solution
|
- In the reaction the major product formed is
Text Solution
|
- In the reaction The major product formed is:
Text Solution
|
- Which of the following reactions involve benzyne intermediate?
Text Solution
|
- In the reaction the major product formed is :
Text Solution
|
- Two benzyne intermediates are likely to be formed equally. Reaction wi...
Text Solution
|
- In the reaction The major product formed is:
Text Solution
|
- In benzyne [] intermediate, the triple bond consists of :
Text Solution
|
- In the reaction the major product formed is :
Text Solution
|
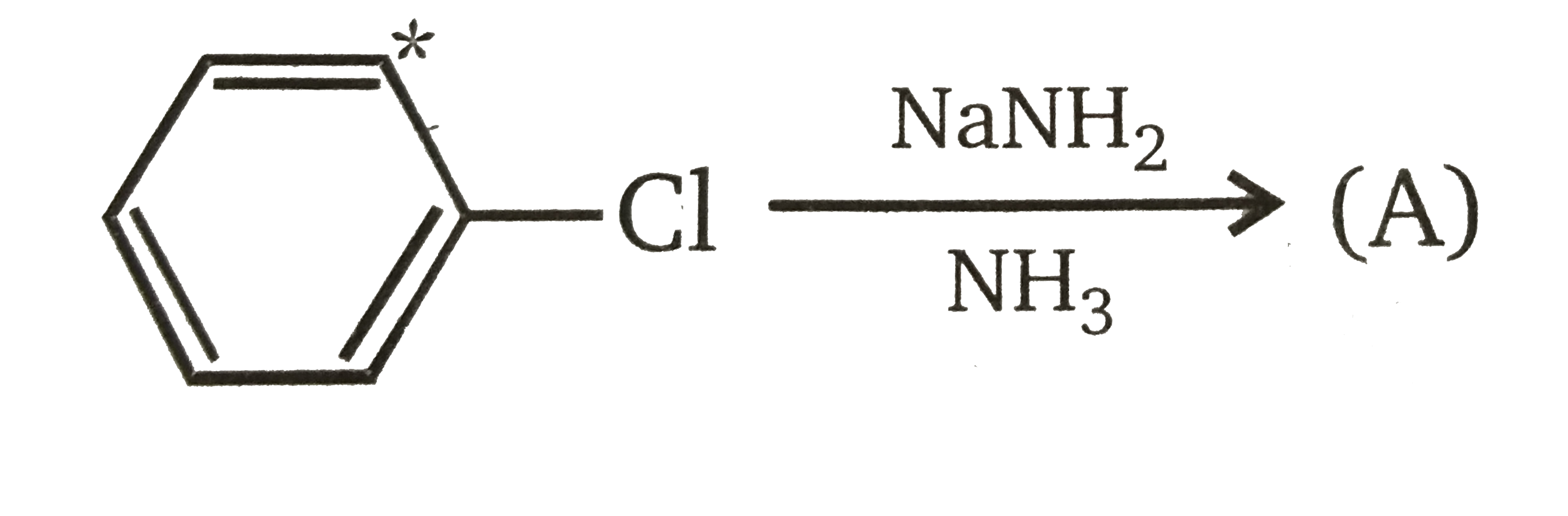 Product major, product (A) is:
Product major, product (A) is: