Similar Questions
Explore conceptually related problems
Recommended Questions
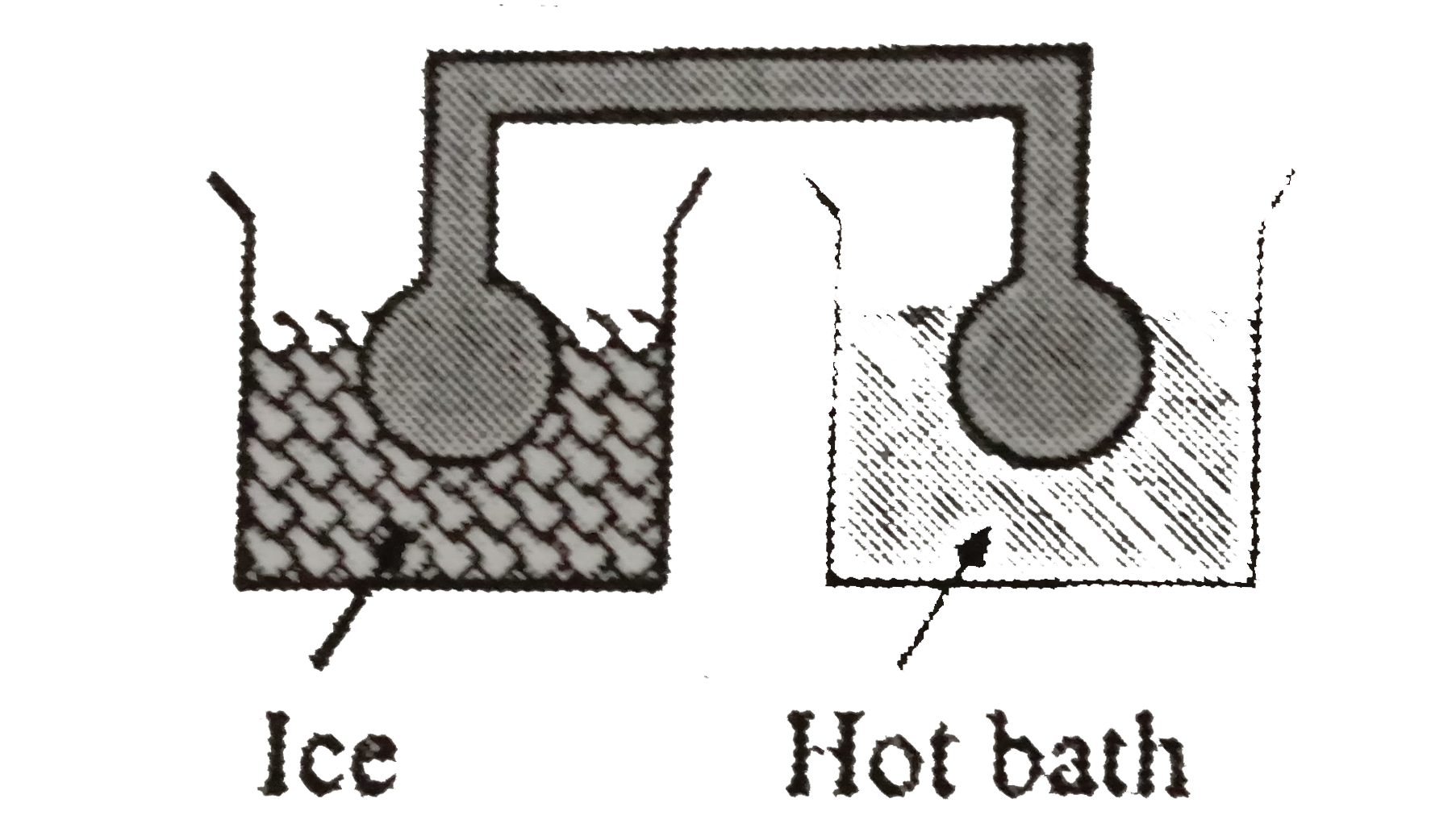
- Two identical glass bulbs are interconnected by a thin glass tube. A g...
Text Solution
|
- two glass bulbs of equal volume are connected by a narrow tube and are...
Text Solution
|
- Two glass bulbs of equal volume are connected by a narrow tube and are...
Text Solution
|
- A gas bulb is filled with NO(2) gas and immersed in an ice bath at 0^(...
Text Solution
|
- A sample of gas at room temperature is placed in an evacuated bulb of...
Text Solution
|
- Two identical glass bulbs are interconnected by a thin tube of negligi...
Text Solution
|
- The pressure of air in a constant volume gas ther5mometer is 0.8m and ...
Text Solution
|
- The pressure indicated by a constant volume hydrogen theemometer are 2...
Text Solution
|
- Two identical glass bulbs are interconnected by a thin glass tube. A g...
Text Solution
|