Similar Questions
Explore conceptually related problems
Recommended Questions
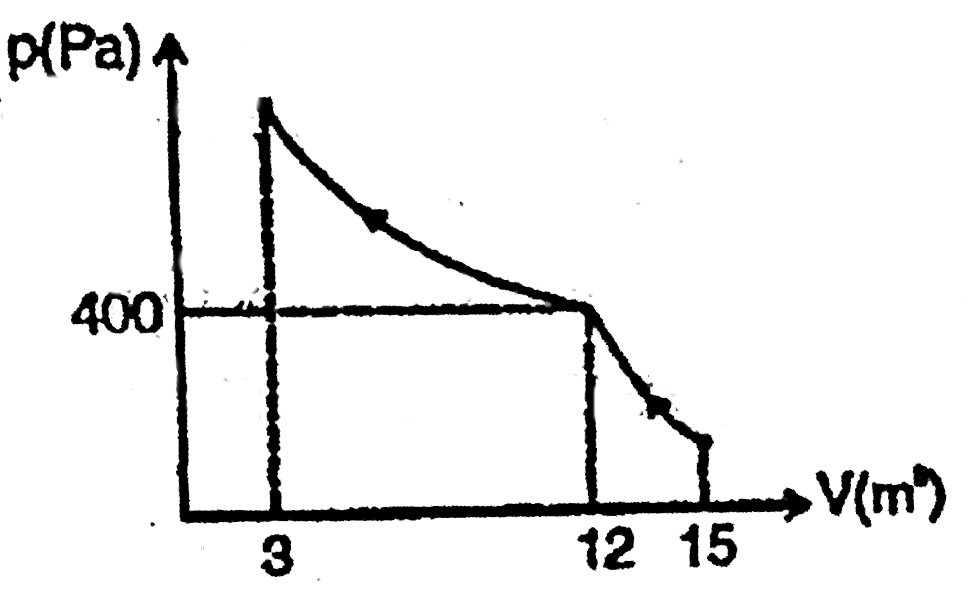
- Curve in the figure shows an adiabatic compression of an ideal gas fro...
Text Solution
|
- An ideal gas expands isothermally from volume V(1) to V(2) and is then...
Text Solution
|
- 200J of work is done to compress an ideal gas isothermally. How much h...
Text Solution
|
- Curve in the figure shows an adiabatic compression of an ideal gas fro...
Text Solution
|
- If an ideal gas is compressed isothermally then
Text Solution
|
- In isothermal ideal gas compression :
Text Solution
|
- If a monoatomic ideal gas of volume 1 litre at N. T.P . Is compressed ...
Text Solution
|
- In isothermal ideal gas compression
Text Solution
|
- An ideal gas of volume V and pressure P expands isothermally to volume...
Text Solution
|