Similar Questions
Explore conceptually related problems
Recommended Questions
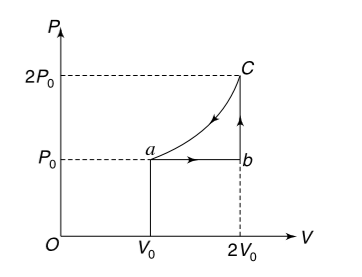
- One mole of an ideal monoatomic gas is taken through a cycle a-b-c-a a...
Text Solution
|
- One mole of an ideal gas is taken through a cyclic process. The minimu...
Text Solution
|
- An ideal monoatomic gas is taken the cycle ABCDA as shown in following...
Text Solution
|
- A sample of an ideal monoatomic gas is taken round the cycle ABCA as s...
Text Solution
|
- One mole of an ideal monoatomic gas is taken through a cycle a-b-c-a a...
Text Solution
|
- One mole of an ideal gas is carried through a thermodynamics cycle as ...
Text Solution
|
- An ideal gas undergoes a circular cycle as shown in the figure. Find t...
Text Solution
|
- One mole of an ideal monoatomic gas is caused to go through the cycle ...
Text Solution
|
- One mole of an ideal monoatomic gas is caused to go through the cycle ...
Text Solution
|