Similar Questions
Explore conceptually related problems
Recommended Questions
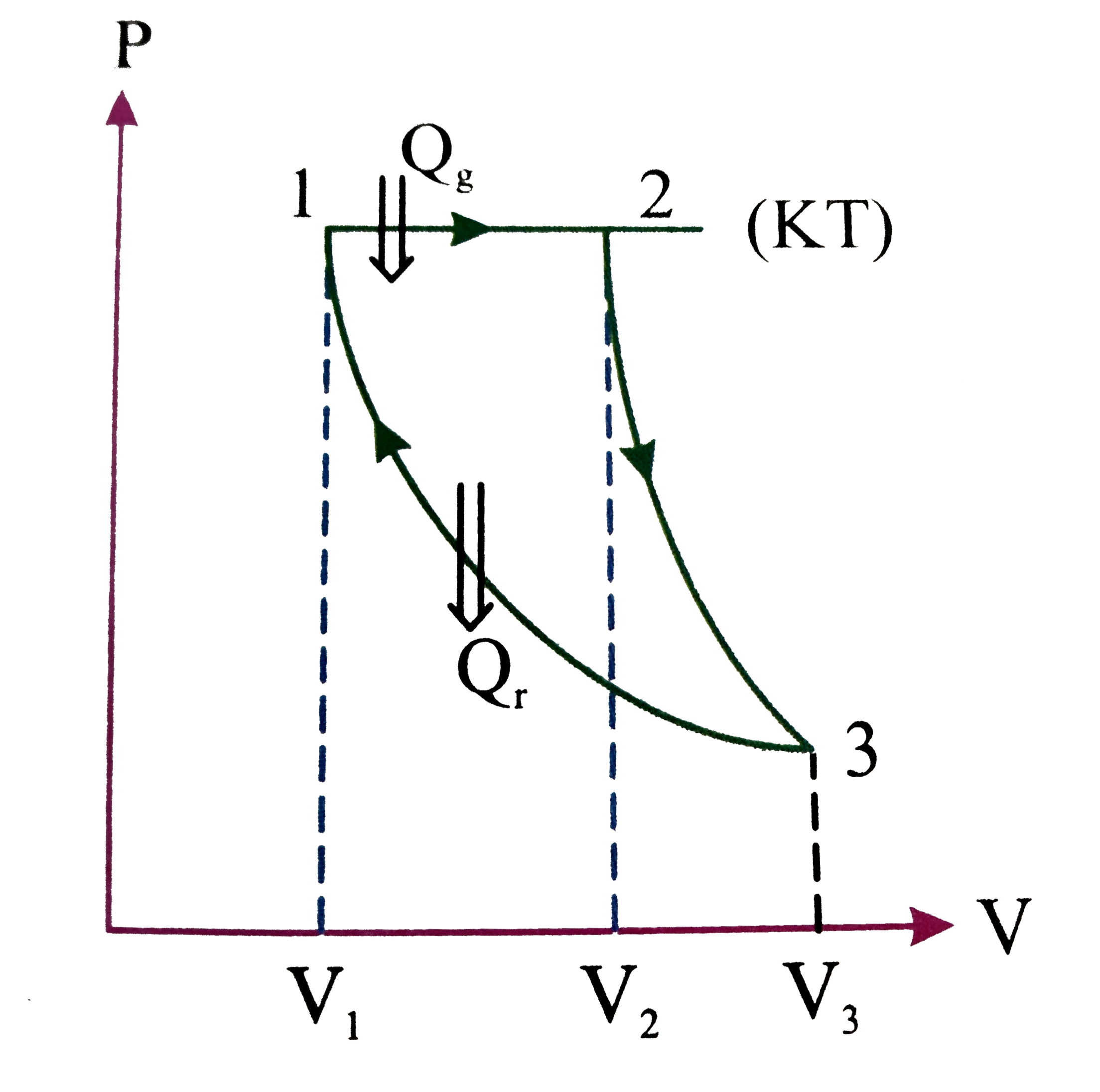
- A cycle is made of three process is iso barie, adiabatic and isotherma...
Text Solution
|
- An ideal gas goes through a cycle consisting of alternate isothermal a...
Text Solution
|
- An ideal gas goes through a cycle consisting of (a) isochoric, adiabat...
Text Solution
|
- An ideal gas goes through a cycle consisting of isothermal, polytropic...
Text Solution
|
- Find the efficiency of a cycle consisting of two isochoric and two iso...
Text Solution
|
- Find the efficiency of a cycle consisting of two isobaric and two isot...
Text Solution
|
- An ideal gas goes through a cycle consisting of ischoric adiabatic and...
Text Solution
|
- A cycle is made of three process is iso barie, adiabatic and isotherma...
Text Solution
|
- One mole of an ideal gas is carried through a thermodynamics cycle as ...
Text Solution
|