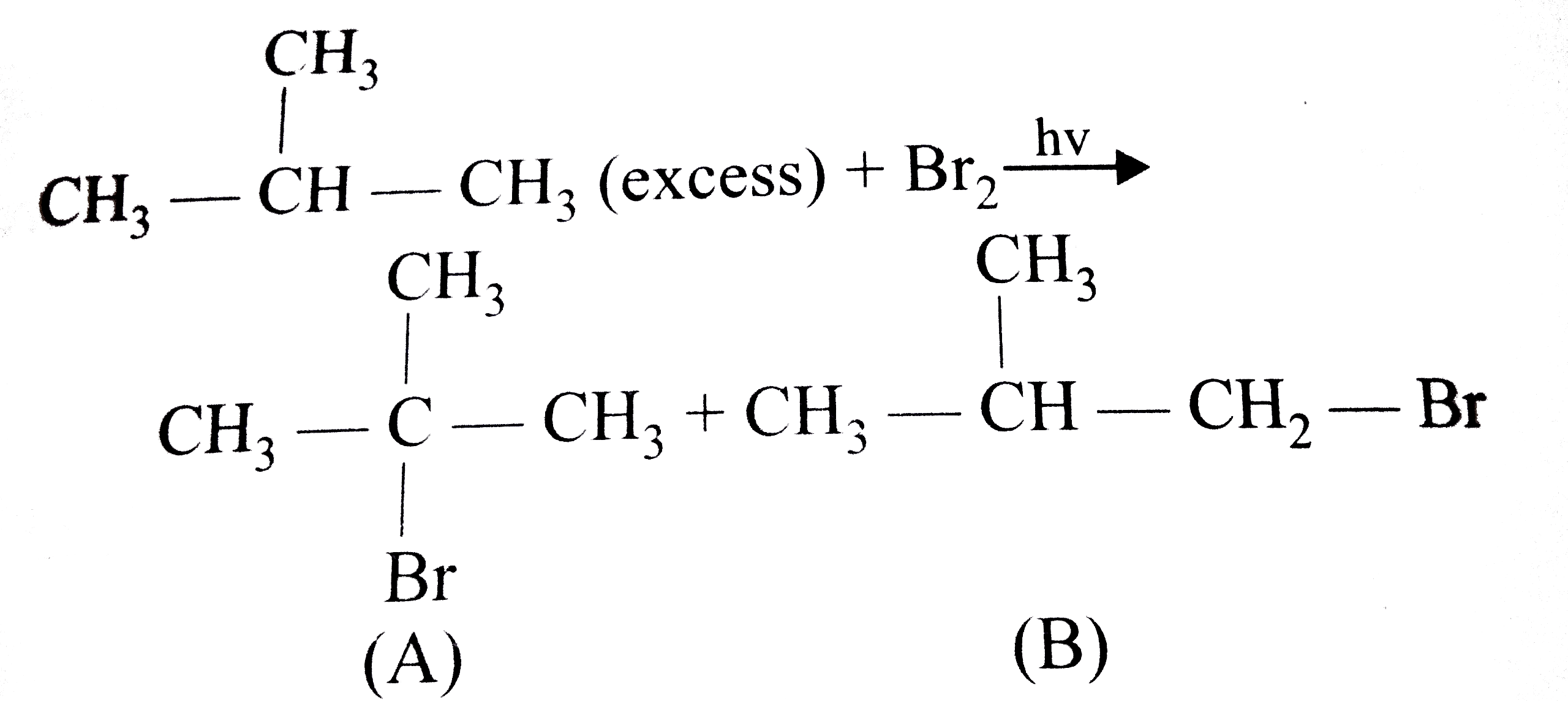Similar Questions
Explore conceptually related problems
Recommended Questions
- The relative reactivity of 1^(@)H,2^(@)H and 3^(@)H in bromination rea...
Text Solution
|
- Calculate the percentage of compounds obtained by monobromination of i...
Text Solution
|
- Bond energy of N-H,H-H and N-=N are a,b,c respectively. The DeltaH for...
Text Solution
|
- The relative reactivity of 1^(@)H,2^(@)H and 3^(@)H in bromination rea...
Text Solution
|
- The relative reactivity of 1^(@) : 2^(@) : 3^(@) H's to brominate is ...
Text Solution
|
- The relative reactivity of 1^(@)H,2^(@)H and 3^(@)H in bromination rea...
Text Solution
|
- The relative reactivity of 1^(@),2^(@) and 3^(@) hydrogens in chlorina...
Text Solution
|
- The relative of 1^(@):2^(@):3^(@) hydrogen to bromination is 1:8...
Text Solution
|
- Calculate the % yield of monochloro products when isopentane is chlori...
Text Solution
|