Similar Questions
Explore conceptually related problems
Recommended Questions
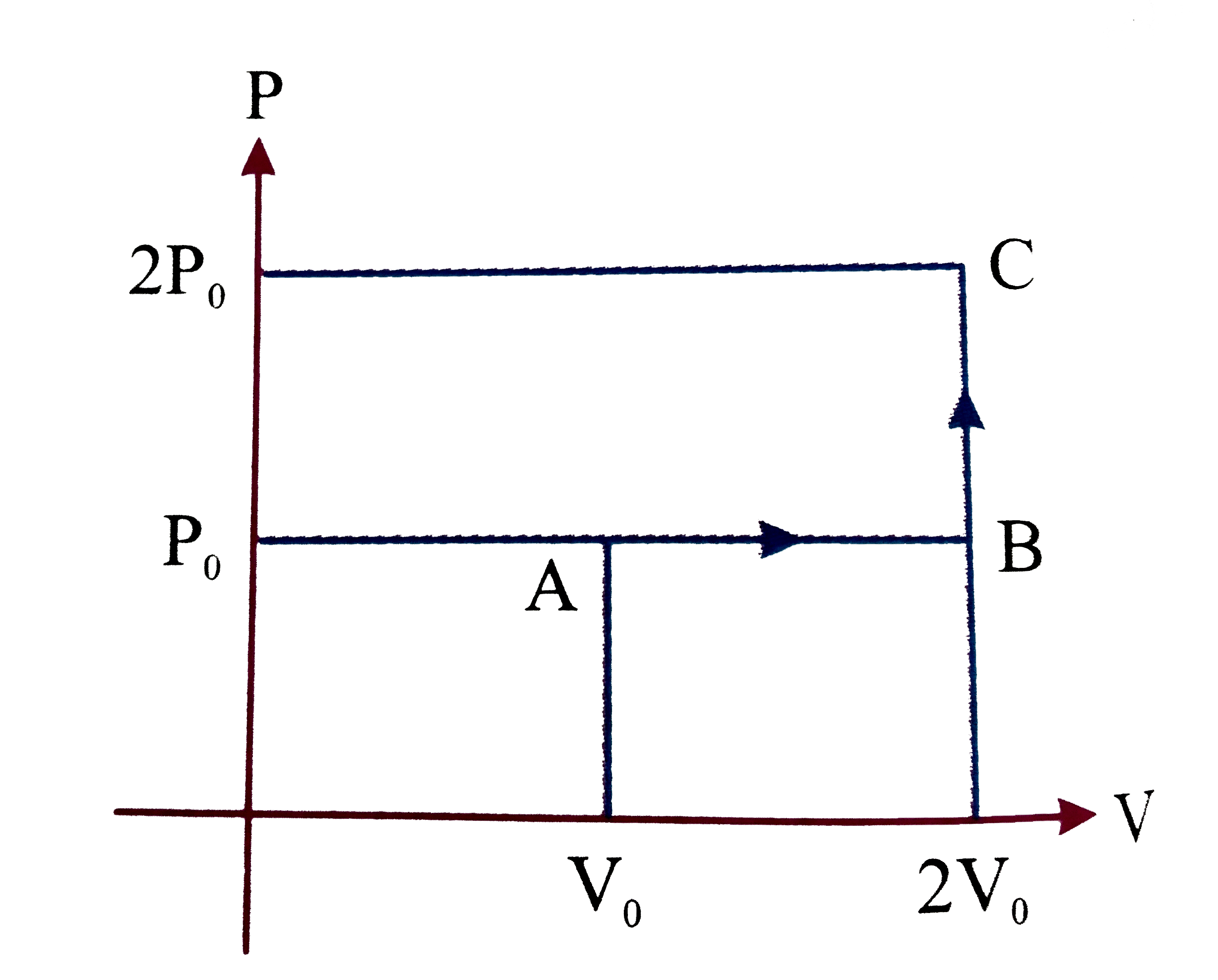
- One mole of an ideal monoatomaic gas is taken from A to C along the pa...
Text Solution
|
- One mole of a monatomic gas is taken from a point A to another point B...
Text Solution
|
- One mole of an ideal monoatomaic gas is taken from A to C along the pa...
Text Solution
|
- In the figure n mole of a monoatomic ideal gas undergo the process ABC...
Text Solution
|
- In the figure n mole of a monoatomic ideal gas undergo the process ABC...
Text Solution
|
- In the figure n mole of a monoatomic ideal gas undergo the process ABC...
Text Solution
|
- One mole of an ideal monoatomic gas undergoes a cyclic process as show...
Text Solution
|
- Two moles of a monoatomic gas are taken from a to c, via three paths a...
Text Solution
|
- One mole of an ideal monoatomic gas is taken along the path ABCA as sh...
Text Solution
|