Similar Questions
Explore conceptually related problems
Recommended Questions
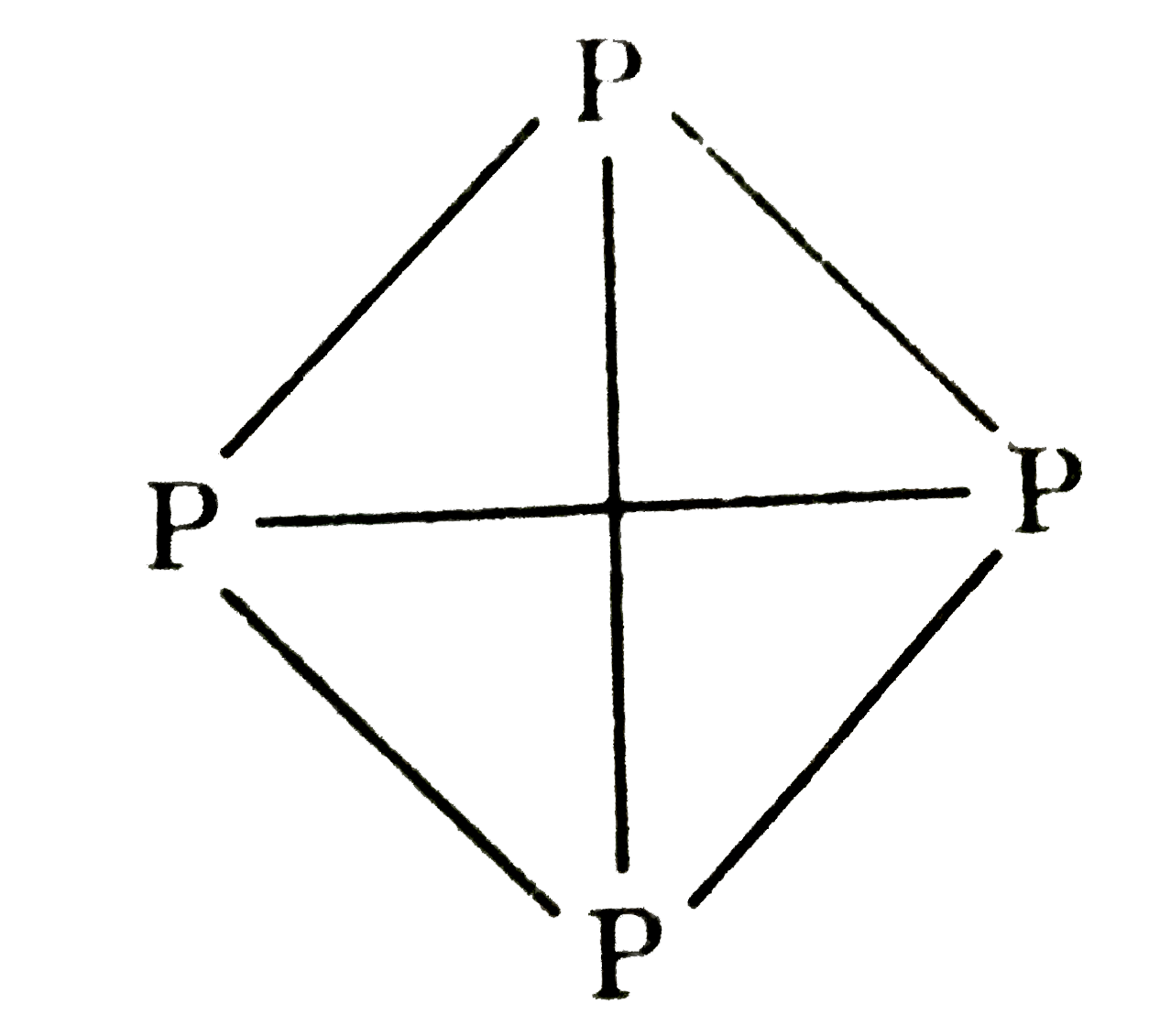
- White phosphorus is a tetra-atomic solid P(4)(s)at room temperature. ...
Text Solution
|
- White phosphorus is a tetra atomic solid P(4) (s) at room temperature....
Text Solution
|
- White phosphorus is a tetra atomic solid [P(4)(s)] at room temperature...
Text Solution
|
- White phosphorus is a tetra atomic solid [P(4)(s)] at room temperature...
Text Solution
|
- What is heat of submisation of P(4)O(6)(s)? Given heat of sublimatio...
Text Solution
|
- Find bond enthalpy of C=O (in kJ/mol) using following information : ...
Text Solution
|
- White phosphorus is a tetra-atomic solid P(4)(s)at room temperature. ...
Text Solution
|
- The heat of atomisation of PH(3)(g) and P(2)H(4)(g) are 954 kJ mol^(-1...
Text Solution
|
- Calculate lattice energy for the change, Li^(+)(g)+Cl^(-) (g) rarr L...
Text Solution
|