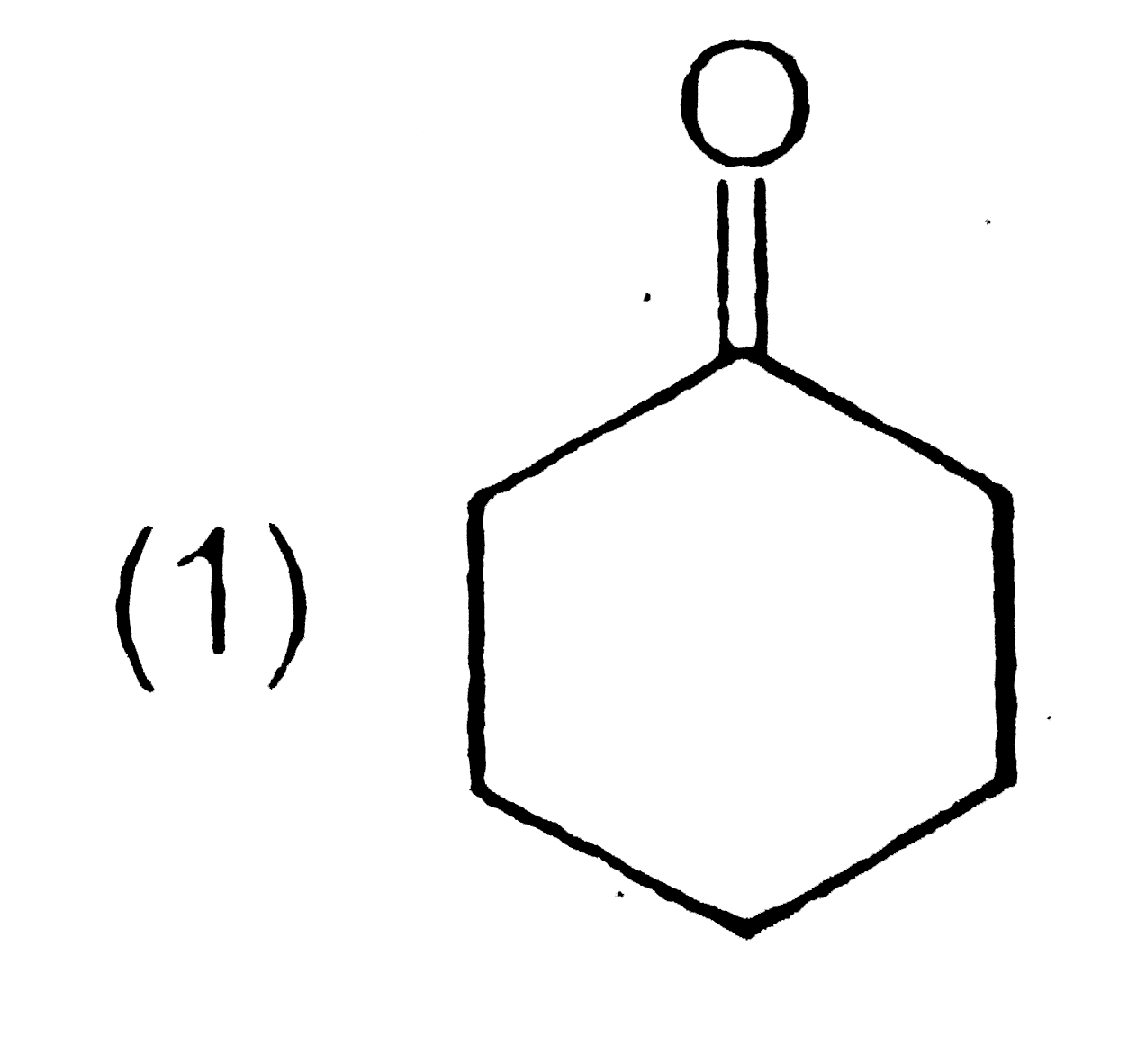
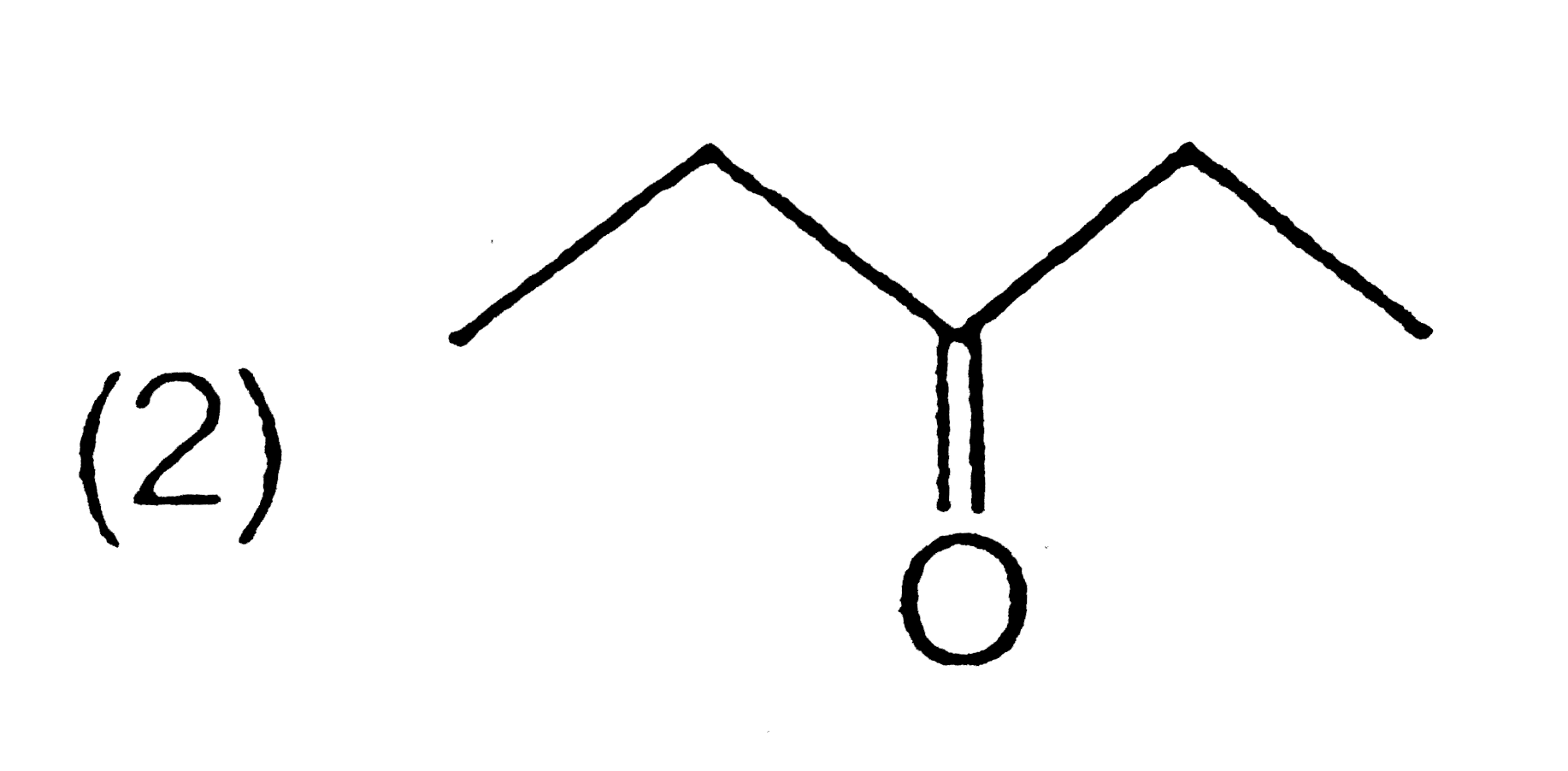
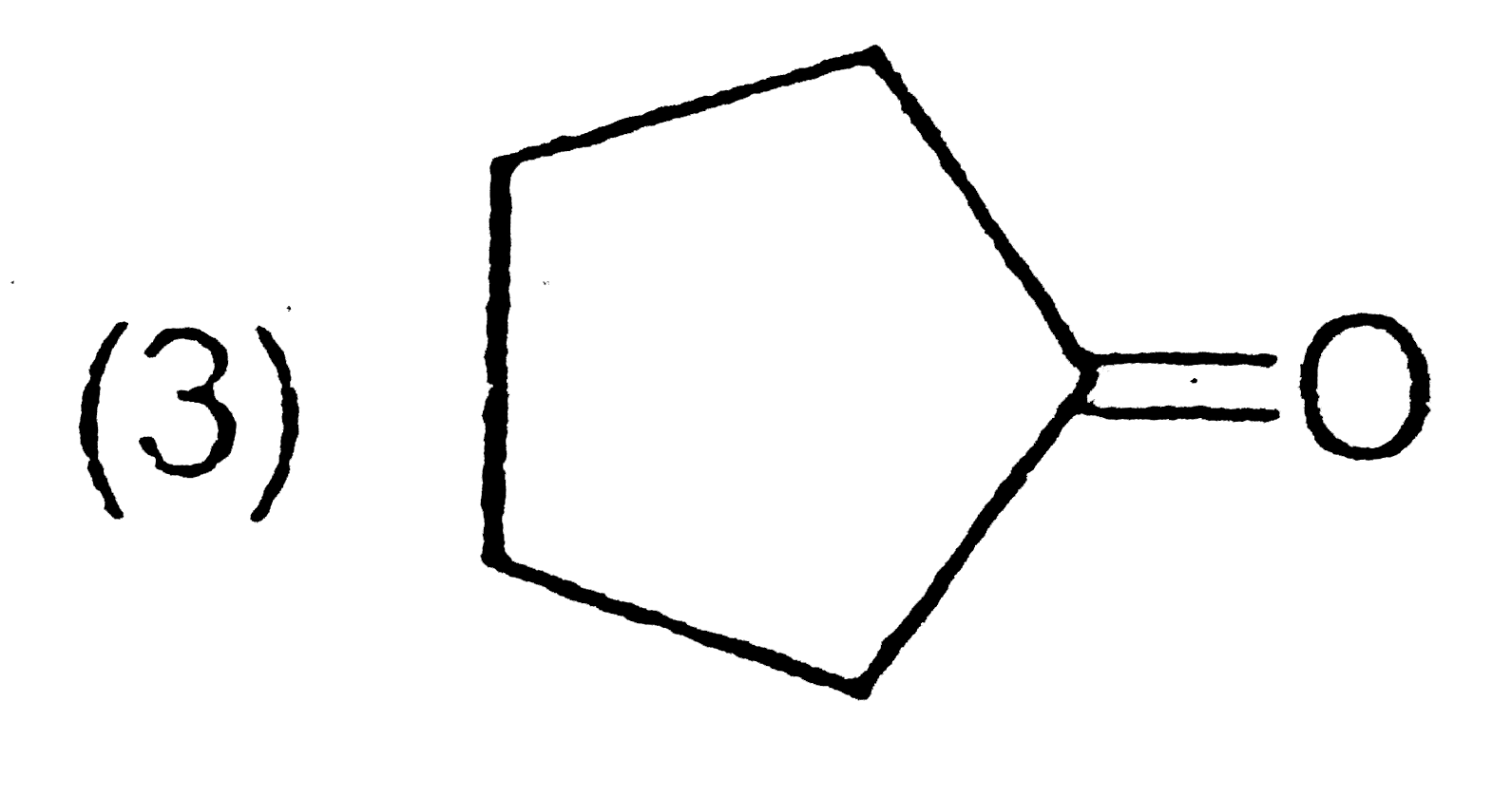
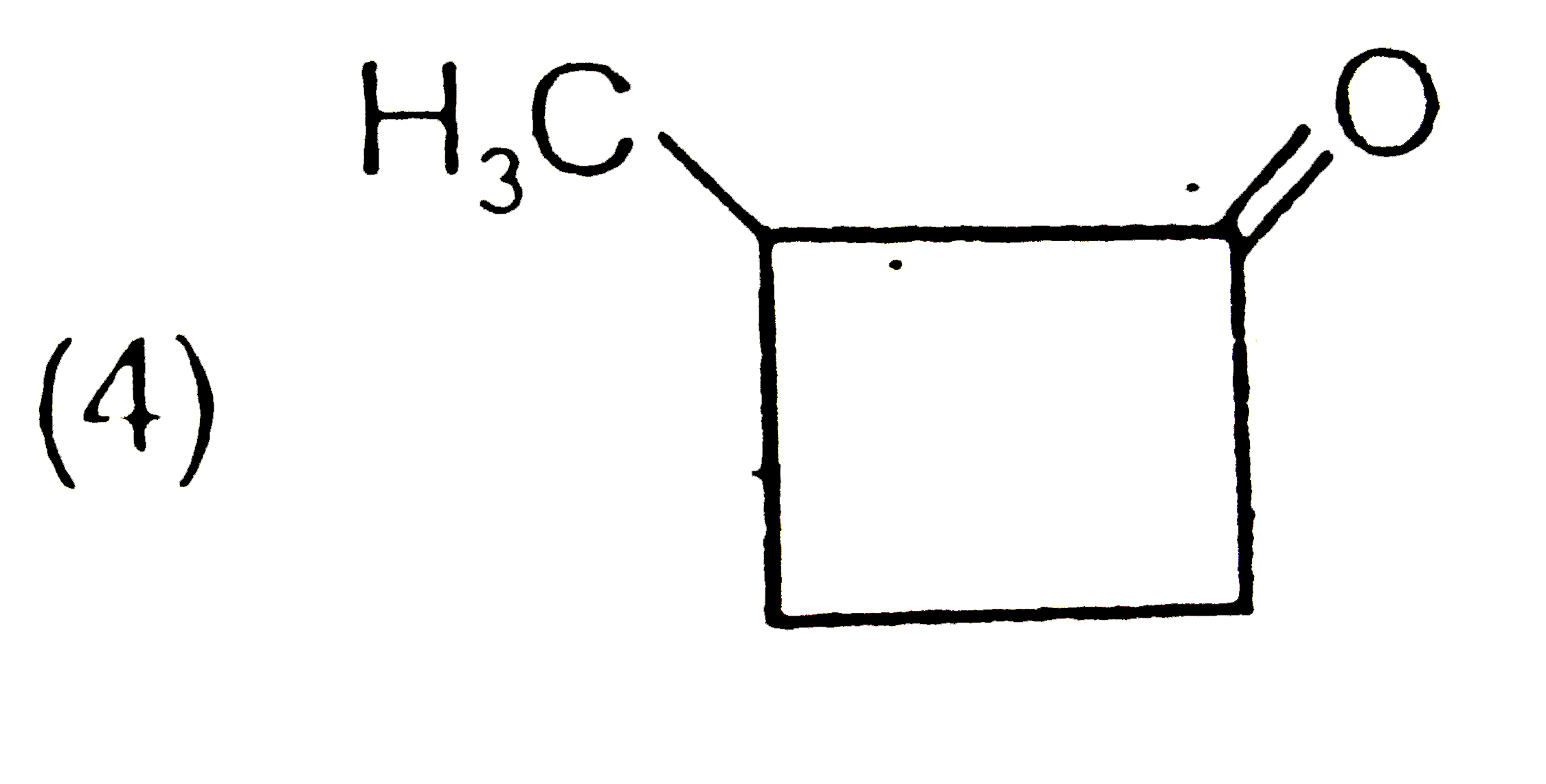
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE|Exercise Assignment (SECTION -C Objective type questions more than one options are correct)|11 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE|Exercise Assignment (SECTION -D Linked Comprehension type Questions)|6 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE|Exercise Assignment (Section A Competition Level Questions)|60 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH INSTITUTE|Exercise Try Yourself|5 VideosAMINES
AAKASH INSTITUTE|Exercise Assignment Section -J (Aakash Challengers Questions)|9 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-ALDEHYDES, KETONES AND CARBOXYLIC ACIDS -Assignment (SECTION -B OBJECTIVE TYPES QUESTIONS (ONE OPTION IS CORRECT)
- Consider the following sequence of reactions: The final product [...
Text Solution
|
- Consider the following sequence of reaction The final product [B]...
Text Solution
|
- CH(3)-overset(O)overset(||)C-CH(2)overset("Conc")underset(HNO(3))to Pr...
Text Solution
|
Text Solution
|
Text Solution
|
Text Solution
|
- The product predominates is
Text Solution
|
- Which of the following carbonyl oxygen will form strongest hydrogen bo...
Text Solution
|
- Which of the following would be the best synthesis of benzoic acid fro...
Text Solution
|
- Consider the following sequence of reactions: Major product [B] o...
Text Solution
|
- The compound which is not reduced by LiAIH(4) is
Text Solution
|
- Consider the following sequence of reactions. The product [A] and...
Text Solution
|
- The compound can be compounded oxidized into
Text Solution
|
- Which of the following conversion is known as Stefen's reduction?
Text Solution
|
- Which reagent or sequence of reagents would best accomplish the follow...
Text Solution
|
- An organic compound [X]. C(5)H(8)O reacts with hydroxylamine to form [...
Text Solution
|
- Alanine can be obtained from acetaldehyde by the following sequence of...
Text Solution
|
- Which of the following carboxylic acid ismost reluctant to form ester ...
Text Solution
|
- Among the given compounds
Text Solution
|
- A is
Text Solution
|