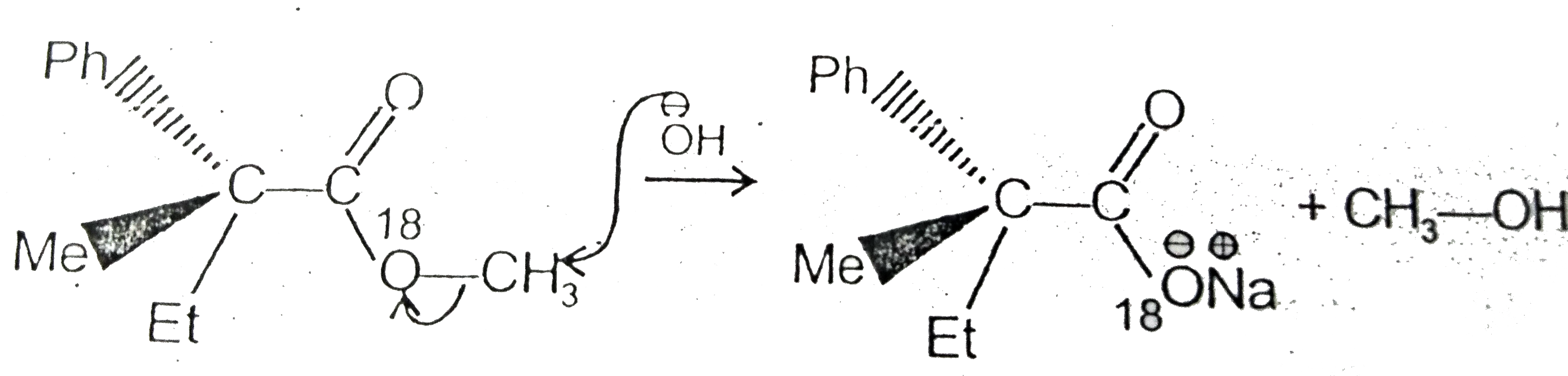
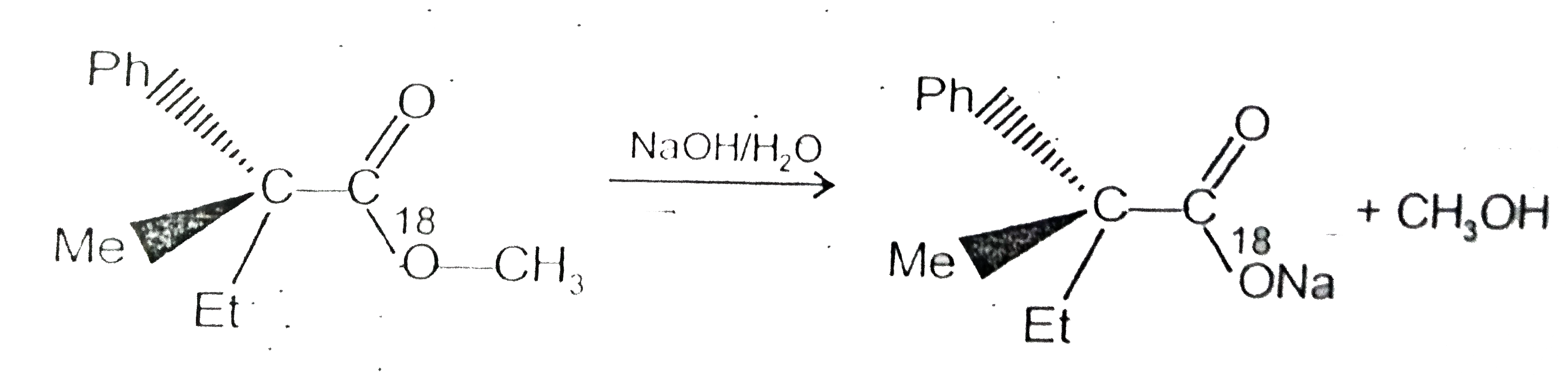Text Solution
Verified by Experts
Topper's Solved these Questions
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE|Exercise Assignment (SECTION -J Akash challengers Questions)|7 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE|Exercise Try Yourself|20 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
AAKASH INSTITUTE|Exercise Assignment (SECTION -H Multiple True-False Type Questions)|2 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH INSTITUTE|Exercise Try Yourself|5 VideosAMINES
AAKASH INSTITUTE|Exercise Assignment Section -J (Aakash Challengers Questions)|9 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-ALDEHYDES, KETONES AND CARBOXYLIC ACIDS -Assignment (SECTION -I Subjective Type Questions)
- Show how you would synthesize each compounds from starting materials c...
Text Solution
|
- Predict the products of the following reactions (a) (b) CH(3)-ove...
Text Solution
|
- Propose mechanism for the given reaction:
Text Solution
|
- Supply the structures of A and B
Text Solution
|
- An organic compound [A] C(9)H(12) gave (B) C(8)H(6)O(4) on oxidation b...
Text Solution
|
- Propose a synthesis of each of the following compounds from the indica...
Text Solution
|
- How many products are formed when compound [X] is decarboxylated?
Text Solution
|
- Suggest a mechanismfor each of the following reactions:
Text Solution
|
- Suggest a synthesis of given product from the indicated starting mater...
Text Solution
|
- Identify the compounds [A] through [D] in the following sequence of re...
Text Solution
|