A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise TEST YOUR GRIP (FILL IN THE BLANKS II)|20 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise CONCEPTUAL QUESTIONS|42 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise EXAMPLE|2 VideosAPPENDIX
PRADEEP|Exercise MODEL TEST PAPER <br> (Section C )|9 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
PRADEEP|Exercise Competition Focus (Jee Main and Advanced / Medical Entrance ) ( Assertion - Reason Type Question ) (Type II)|12 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-CHEMICAL BONDING AND MOLECULAR STRUCTURE-TEST YOUR GRIP (MULTIPLE CHOICE QUESTIONS I)
- Which of the following contains both covalent and ionic bond?
Text Solution
|
- Soperoctet molecule is
Text Solution
|
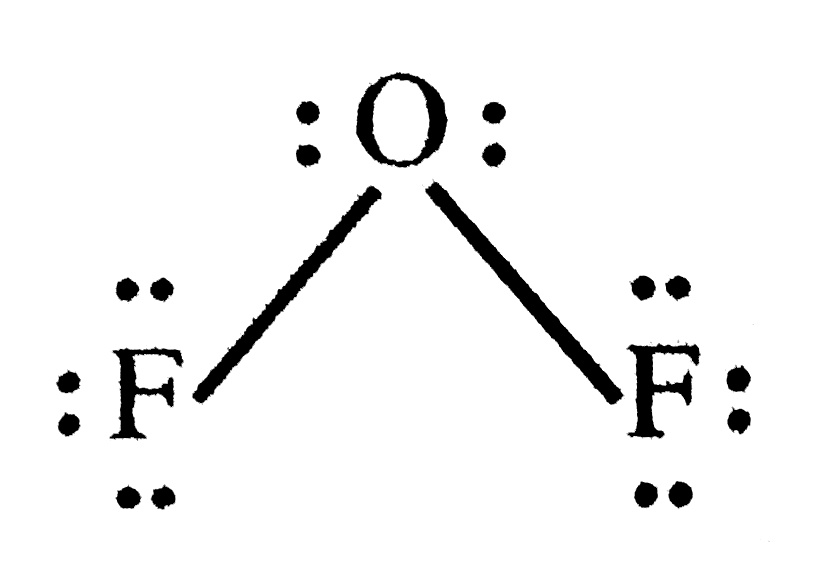
- In OF2, the number of bond pairs and lone pairs of electrons are respe...
Text Solution
|
- When two hydrogen atoms approach each other to form H(2) molecule, t...
Text Solution
|
- The number of sigma and pi- bonds in allyl isocyanide are
Text Solution
|
- Which contains both polar and non-polar bonds ? .
Text Solution
|
- Which one of the following has the highest dipole moment ?
Text Solution
|
- Which of the following hydrocarbons has the lowest dipole moment ?
Text Solution
|
- A neutral molecule XF(3) has a zero diple moment. The element X is mos...
Text Solution
|
- Polarizing power Cd^(2+) on the anions is stronger then that of Ca^(...
Text Solution
|
- In which of the following the central atoms does not use sp^(3) hybrid...
Text Solution
|
- In an octahedral structure , the pair of d orbitals involved in d^(2)...
Text Solution
|
- The correct order regarding the electronegativity of hybrid orbitals o...
Text Solution
|
- The bond angle formed by different hybrid orbitals are in the order
Text Solution
|
- The structure of IF(7) is
Text Solution
|
- Which one of the following molecules has the smallest bond angle ?
Text Solution
|
- Molecular shape of SF(4), CF(4) and XeF(4) are
Text Solution
|
- In BrF(3) molecule, the lone pairs occupy requatorial position to mi...
Text Solution
|
- Which of the following molecular orbitals has two nodal planes ?
Text Solution
|
- Which of the following species does not exist under normal condition...
Text Solution
|