Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise NCERT Questions And Exercises With Answers|39 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise NCERT EXAMPLAR PROBLEMS (MULTIPLE CHOICE QUESTIONS -I)|22 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise TEST YOUR GRIP (FILL IN THE BLANKS II)|20 VideosAPPENDIX
PRADEEP|Exercise MODEL TEST PAPER <br> (Section C )|9 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
PRADEEP|Exercise Competition Focus (Jee Main and Advanced / Medical Entrance ) ( Assertion - Reason Type Question ) (Type II)|12 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-CHEMICAL BONDING AND MOLECULAR STRUCTURE-CONCEPTUAL QUESTIONS
- Why does NaCl give a white precipitate with AgNO(3) solution but C Cl(...
Text Solution
|
- Why reaction between NaCl and AgNO(3) is very fast but reaction bet...
Text Solution
|
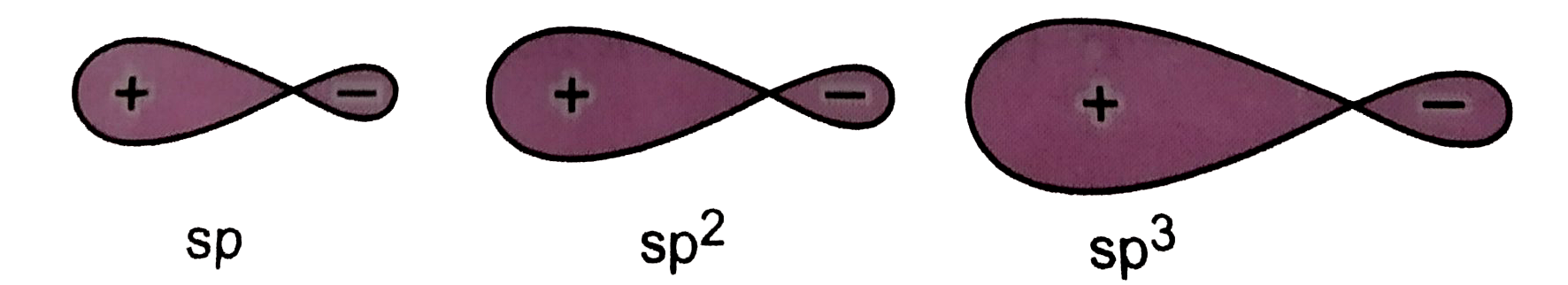
- Draw the shapes of the following hybrid orbitals : sp, sp^(2), sp^(3...
Text Solution
|
- Name the type of hybridisation of each C-atom in a molecule of (i) pro...
Text Solution
|
- Out of p - orbital and sp-hybrid orbital which has greater directions ...
Text Solution
|
- What angles are associated with the following orbitals ? sp,sp^(2)...
Text Solution
|
- Which d-orbital is involved in sp^(3) d hydridisation and why ?
Text Solution
|
- Which d-orbital is involved in dsp^(2) hybridisation why ?
Text Solution
|
- What is the hybrid state of BeCl(2) ? What will be the change in the ...
Text Solution
|
- Arrange the following in order of decreasing bond angles (i)CH(4),...
Text Solution
|
- Write the structure of an anion which is isostructural with BF(3) and...
Text Solution
|
- Why axis bonds of PCl(5) are longer than equatorial bonds ?
Text Solution
|
- Name the different type of bonds present in NH(4) Cl after drawing its...
Text Solution
|
- Write two resonance structure of N2 O that satisfy the octet rule.
Text Solution
|
- Which of the following species have same shape/same bond order ? N...
Text Solution
|
- Taking Z-axis as the internuclear axis, explain why 2p(x) or 2p(y) ...
Text Solution
|
- Compara the relative stablilties of O(2)^(-) and N(2)^(-) and comment...
Text Solution
|
- (a) How bond energy veries from N(2) ^(-) to N(2)^(+) and why ? ...
Text Solution
|
- N(2) has higher order than NO. Explain .
Text Solution
|
- Ethanol has higher boiling point diethyl ether or ethyl amine. Why ?
Text Solution
|

 .
.