Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise NCERT EXAMPLAR PROBLEMS (MULTIPLE CHOICE QUESTIONS -I)|22 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise NCERT EXAMPLAR PROBLEMS (MULTIPLE CHOICE QUESTIONS -II)|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise CONCEPTUAL QUESTIONS|42 VideosAPPENDIX
PRADEEP|Exercise MODEL TEST PAPER <br> (Section C )|9 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
PRADEEP|Exercise Competition Focus (Jee Main and Advanced / Medical Entrance ) ( Assertion - Reason Type Question ) (Type II)|12 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-CHEMICAL BONDING AND MOLECULAR STRUCTURE-NCERT Questions And Exercises With Answers
- H(3) PO(3) can be represented by the structures 1 and 2 shown below . ...
Text Solution
|
- Write the resonance structures for SO(3),NO(2), and NO(3)^(ө).
Text Solution
|
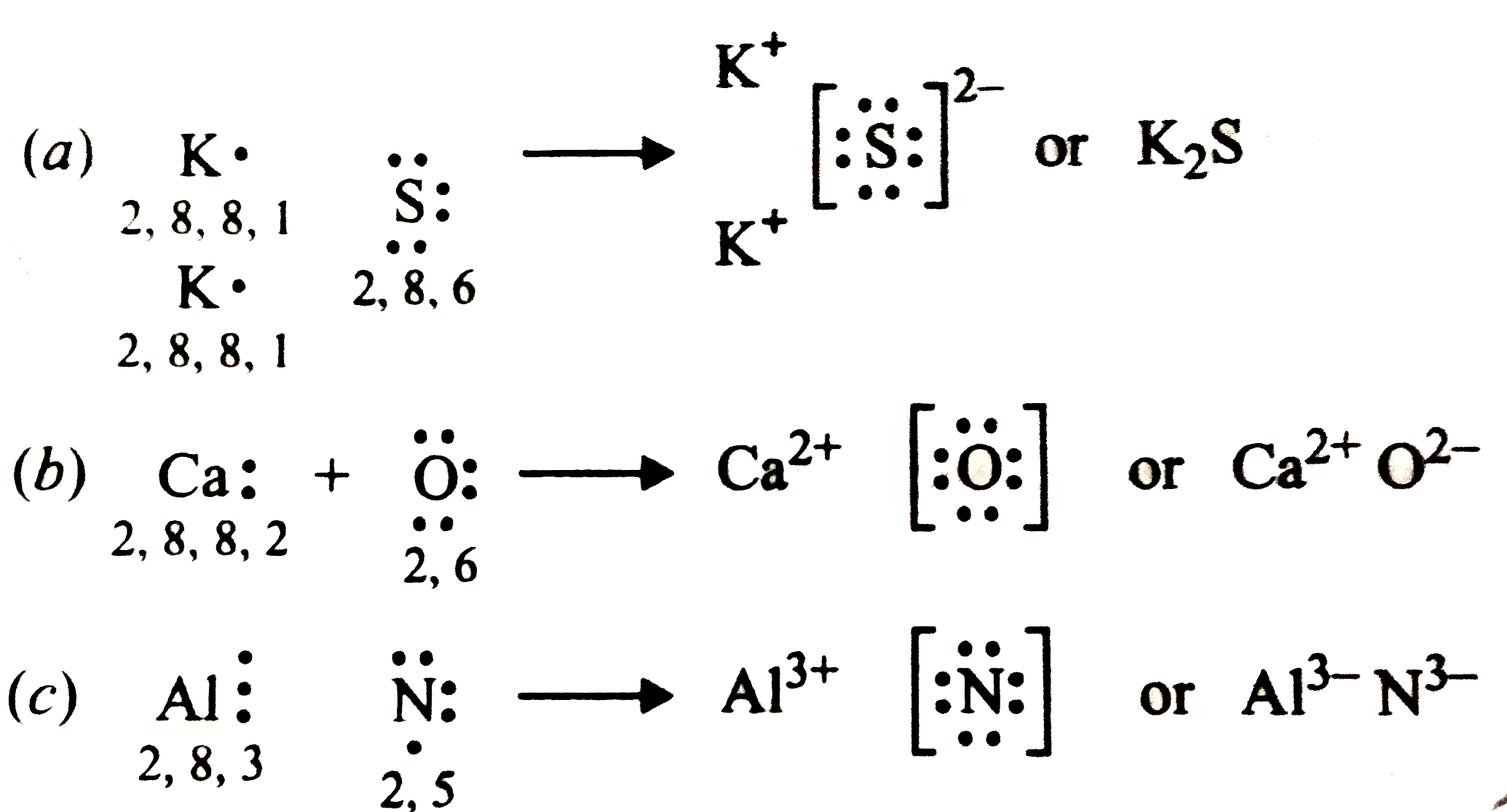
- Use Lewis symbols to show electron transfer between the following atom...
Text Solution
|
- Although both CO(2) and H(2)O are triatomic molecules, the shape of H(...
Text Solution
|
- Write the significance/applications of dipole moment.
Text Solution
|
- Define electronegativity. How does it differ from electron gain enthal...
Text Solution
|
- Explain with the help of suitable example polar covalent bond.
Text Solution
|
- Arrange the following molecules in order ionic character of their bond...
Text Solution
|
- The skeletal structure of CH(3) COOH as shown below is correct , but...
Text Solution
|
- Apart from tetrahedral geometry, another possible geometry for CH(4) ...
Text Solution
|
- Explain why BeH(2) molecule has a zero dipole moment although the Be-H...
Text Solution
|
- Which out of NH(3) and NF(3) has higher dipole ment and why ?
Text Solution
|
- What is meant by hybridisation of atomic orbitals? Describe the shape ...
Text Solution
|
- What is the change in hybridization (if any) of the Al atom in the fol...
Text Solution
|
- Is there any change in hybridisation of the B and N atom as a result o...
Text Solution
|
- Draw diagrams showing the formation of a double bond and a triple bond...
Text Solution
|
- what is the total number of sigma and pi bonds in the following molecu...
Text Solution
|
- Considering x-axis as the internuclear axis, which out of the followin...
Text Solution
|
- Which hybrid orbitals are usel by carbon atoms in the following molecu...
Text Solution
|
- What do you understand by bond pairs and lone pairs of electrons? Illu...
Text Solution
|