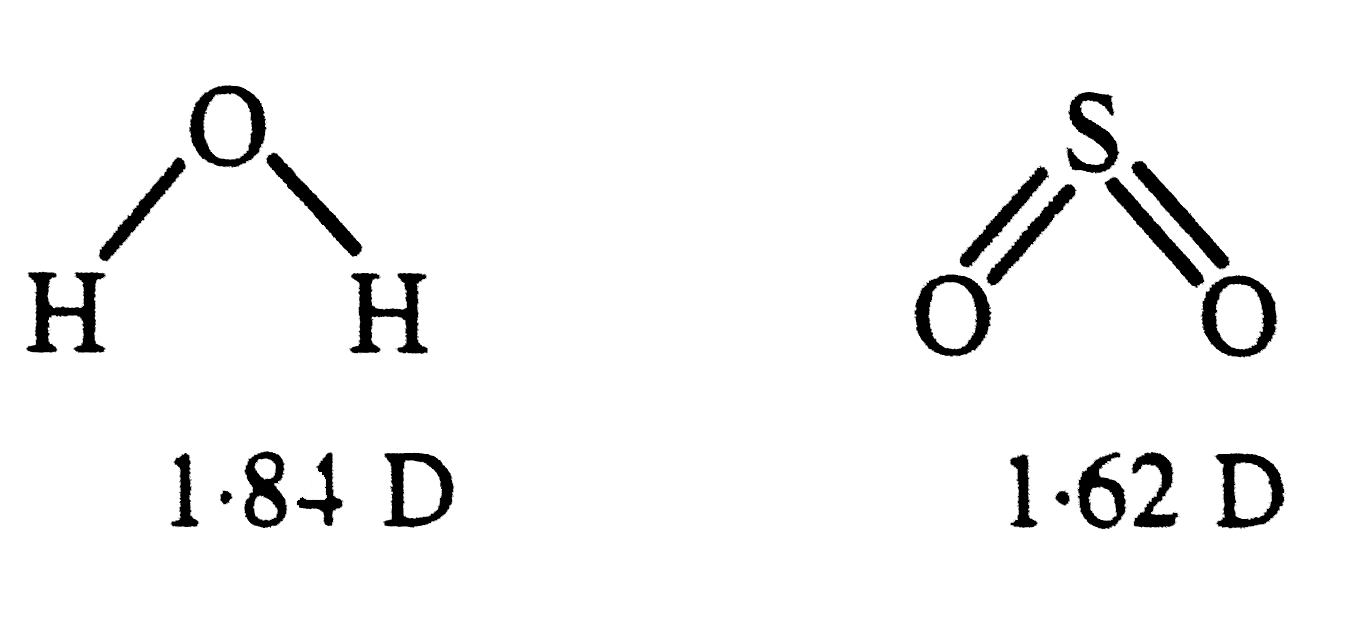A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise NCERT EXAMPLAR PROBLEMS (MULTIPLE CHOICE QUESTIONS -II)|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise NCERT EXAMPLAR PROBLEMS (MULTIPLE CHOICE QUESTIONS -1)|7 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise NCERT Questions And Exercises With Answers|39 VideosAPPENDIX
PRADEEP|Exercise MODEL TEST PAPER <br> (Section C )|9 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
PRADEEP|Exercise Competition Focus (Jee Main and Advanced / Medical Entrance ) ( Assertion - Reason Type Question ) (Type II)|12 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-CHEMICAL BONDING AND MOLECULAR STRUCTURE-NCERT EXAMPLAR PROBLEMS (MULTIPLE CHOICE QUESTIONS -I)
- Isostructrual species are those which have the same shape and hybridis...
Text Solution
|
- Polarity in a molecule and hence the dipole moment depends primarily o...
Text Solution
|
- The types of hybrid orbitals of nitrogen in NO(2)^(+),NO(3)^(-) and NH...
Text Solution
|
- Hydrogen bonds are formed in many compounds e.g. H(2)O, HF, NH(3). The...
Text Solution
|
- In PO(4)^(3-) ion, the formal charge on the oxygen atom of P-O bond...
Text Solution
|
- In NO(3)^(-) ion, the number of bond pairs and lone pairs of electrons...
Text Solution
|
- Which of the following species has tetrahedral geometry?
Text Solution
|
- Number of pi bonds and sigma bonds in the following structer is
Text Solution
|
- Which molecule/ion out of the following does not contain unpaired elec...
Text Solution
|
- In which of the following molecule/ion all the bonds are not equal?
Text Solution
|
- In which of the following substance will hydrogen bond be strongest...
Text Solution
|
- If the electron configuration of an element is 1s^(2), 2s^(2), 2p^(6),...
Text Solution
|
- Which of the following angle corresponds to sp hydridisation ?
Text Solution
|
- The electronic configurations of three elements, A,B are C are give...
Text Solution
|
- The electronic configuration ofhte elements. A, B and C are given belo...
Text Solution
|
- The electronic configuration ofhte elements. A, B and C are given belo...
Text Solution
|
- The electronic configurations of three elements, A,B are C are give...
Text Solution
|
- Which of the following order of energies of molecular orbitals of N(2...
Text Solution
|
- Which of the following statement is not correct from the view point of...
Text Solution
|
- Which of the following options represents the correct bond order ?
Text Solution
|