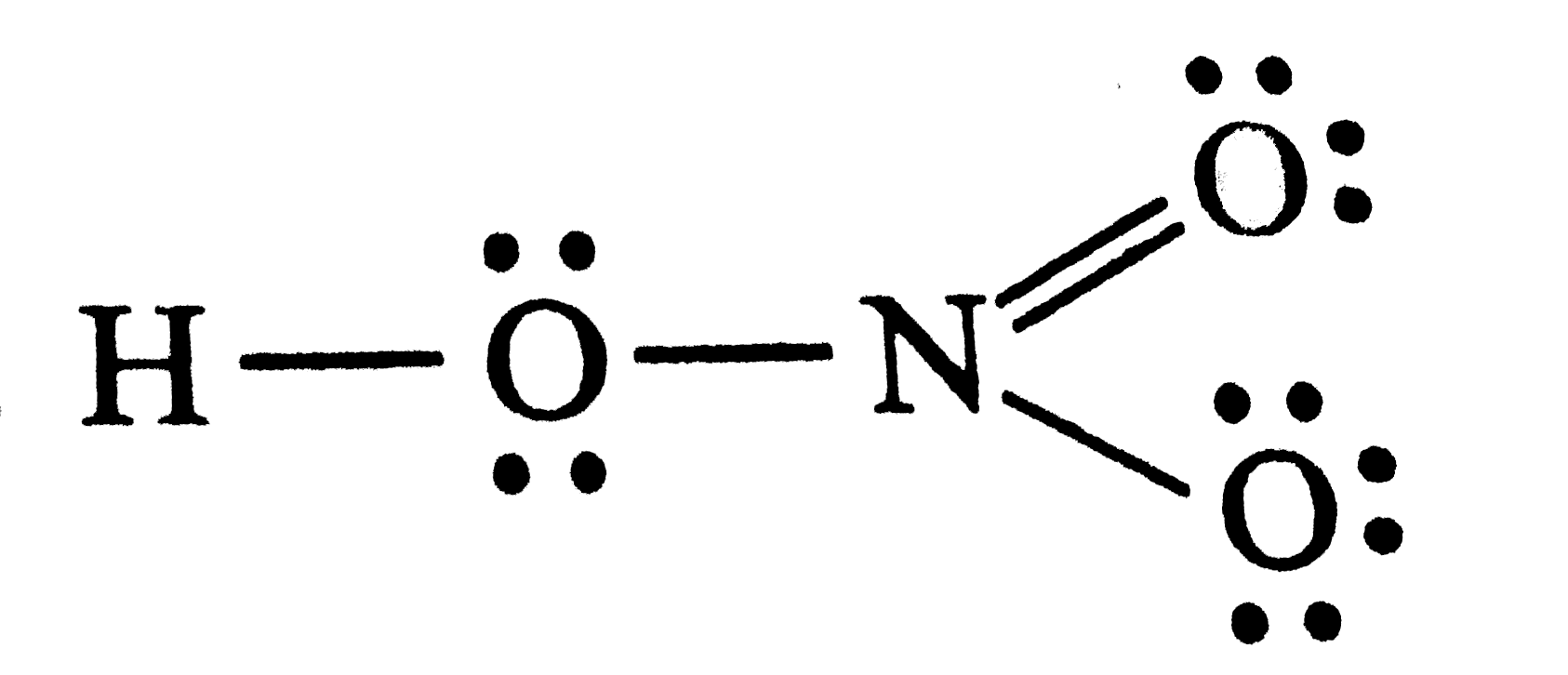Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise COMPETITION FOCUS JEE (Main and Advanced)/ MEDICAL ENTRANCE SPECIAL (VII. NUMERICAL VALUE TYPE QUESTIONS (IN DECIMAL NOTATION))|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise COMPETITION FOCUS JEE (Main and Advanced)/ MEDICAL ENTRANCE SPECIAL (VIII. ASSERTION-REASON TYPE QUESTIONS TYPE I)|30 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
PRADEEP|Exercise COMPETITION FOCUS JEE (Main and Advanced)/ MEDICAL ENTRANCE SPECIAL (V MATRIX-MATCH TYPE QUESTIONS)|3 VideosAPPENDIX
PRADEEP|Exercise MODEL TEST PAPER <br> (Section C )|9 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
PRADEEP|Exercise Competition Focus (Jee Main and Advanced / Medical Entrance ) ( Assertion - Reason Type Question ) (Type II)|12 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-CHEMICAL BONDING AND MOLECULAR STRUCTURE-COMPETITION FOCUS JEE (Main and Advanced)/ MEDICAL ENTRANCE SPECIAL (VI. INTEGER TYPE QUESTIONS)
- In Al2Cl6 each Al atoms is linked to how many Cl atoms ?
Text Solution
|
- Total number of lone pairs present in the structure of HNO(3) is
Text Solution
|
- Total number of electron pairs (both lone and bond pairs) around centr...
Text Solution
|
- Total number of molecular orbitals occupying one or two electrons in O...
Text Solution
|
- The number of 90^(@) bond angles present in SF(4) molecules is
Text Solution
|
- Total number of sigma-bond present in the molecula of propyne is
Text Solution
|
- Total number of coordinate bonds present in CuSO(4).5H(2)O is
Text Solution
|
- Number of H(2) molecules attached to each H(2)O molecule through hydro...
Text Solution
|
- The number of water molecule(s) directly bonded to the metal centre in...
Text Solution
|
- Based on VSEPR theory, the number of 90 degree F-Br-F angles in BrF(5)...
Text Solution
|
- A list of species having the formula of XZ(4) is given below XeF(4), S...
Text Solution
|
- The total number of lone pair of electrons in N(2)O(3) is
Text Solution
|
- Among the triatomic molecules/ions BeCl(2),N(3)^(-),N(2)O, NO(2)^(+), ...
Text Solution
|
- The sum of the number of lone pair of electrons on each central atom i...
Text Solution
|
- Among H(2), He(2)^(+), Li(2), Be(2), B(2), C(2), N(2), O(2)^(-) and F(...
Text Solution
|