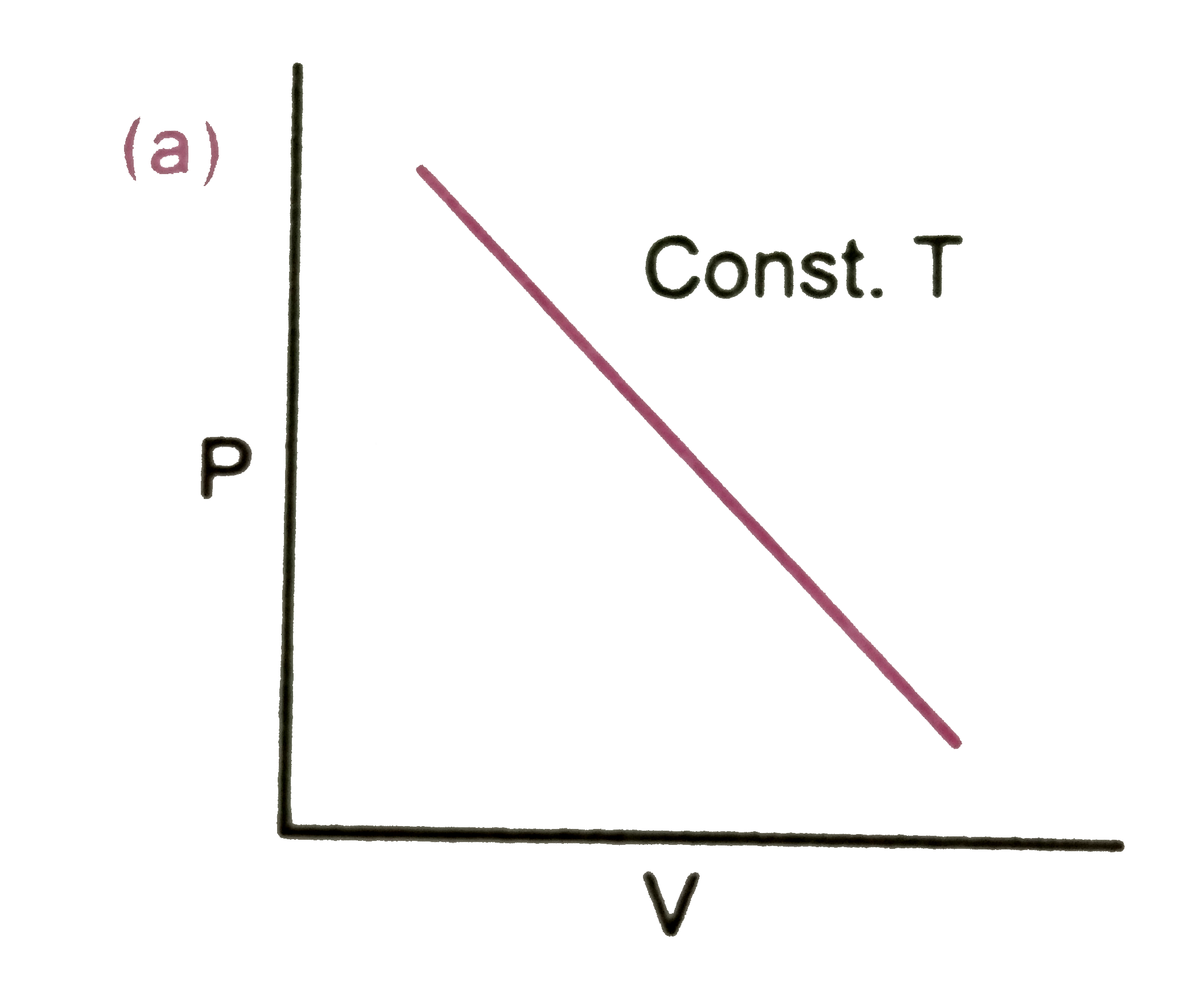
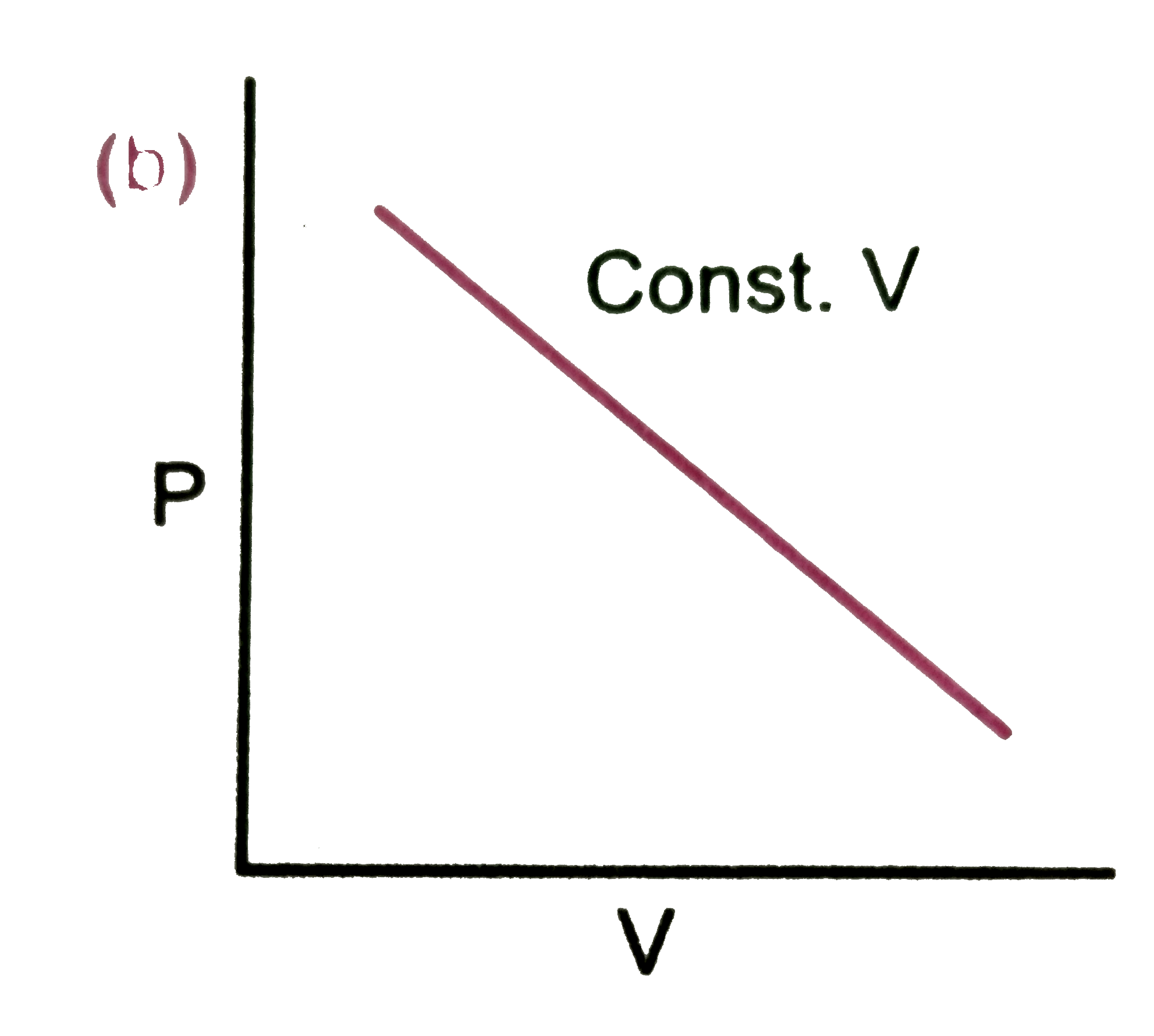
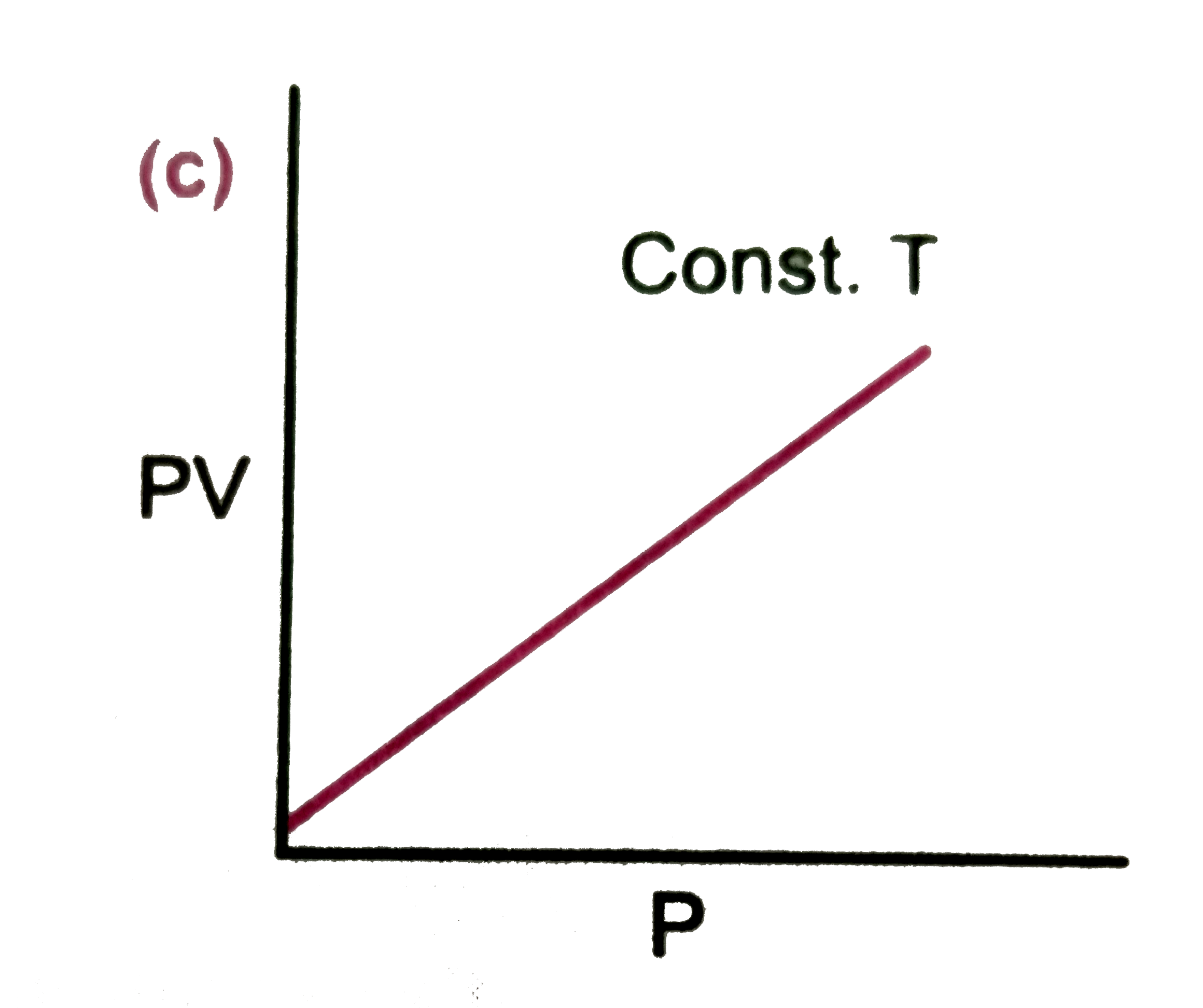
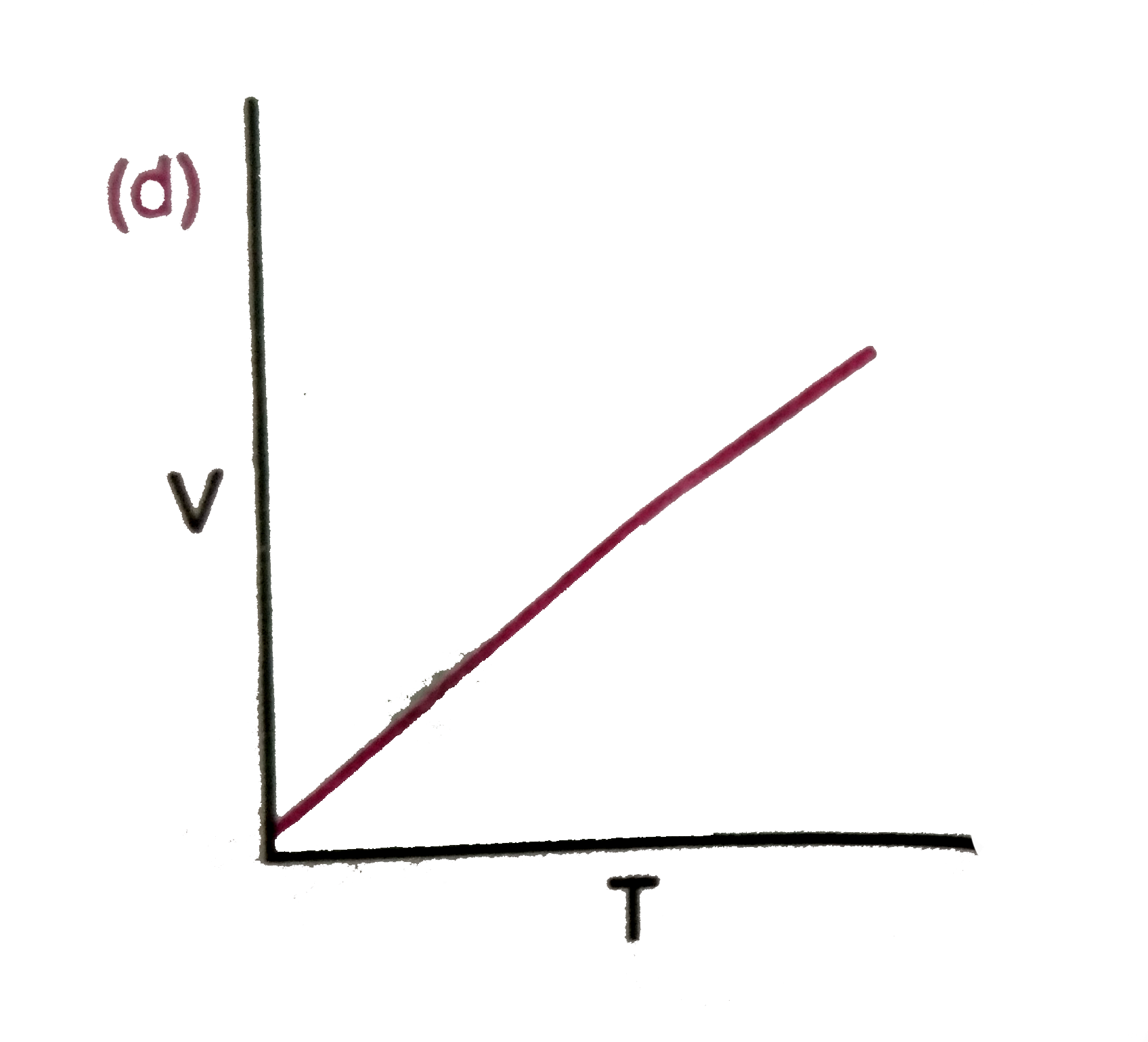
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER : GASES AND LIQUIDES
PRADEEP|Exercise FILL IN THE BLANK|20 VideosSTATES OF MATTER : GASES AND LIQUIDES
PRADEEP|Exercise CONCEPTUAL QUESTIONS (Differentiating three states of matter and intermolecular forces|2 VideosSTATES OF MATTER : GASES AND LIQUIDES
PRADEEP|Exercise ADVANCED PROBLEMS (FOR COMPETITIONS)|19 VideosSOME p-BLOCK ELEMENTS
PRADEEP|Exercise Competition Focus (JEE( Main and Advanced)/Medical Entrance) VIII. Assertion-Reason Type Questions (Type I)|23 VideosSTATES OF MATTER: SOLID MATTER
PRADEEP|Exercise COMPETITION FOCUS (ASSERTION-REASON)|17 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-STATES OF MATTER : GASES AND LIQUIDES-TEST YOUR GRIP
- When two ice cubes are pressed over each other, they unite to form one...
Text Solution
|
- The slope of the plot between pV and p at constant temperature is
Text Solution
|
- Which of the following diagram correctly describes the behaviour of a ...
Text Solution
|
- If 300 ml of a gas weigh 0.368 g at STP, what is its molecular weight ...
Text Solution
|
- The density of neon will be highest at
Text Solution
|
- Gas equation PV = nRT is obeyed by
Text Solution
|
- Molar volume of CO(2) is maximum at
Text Solution
|
- The density of a gas a is twice that of gas B. Molecular mass of A is ...
Text Solution
|
- According to Graham's law, at a given temperature, the ratio of the ra...
Text Solution
|
- Two gases A and B having the same volume diffuse through a porous part...
Text Solution
|
- Which of the following pairs will effuse at the same rate through a po...
Text Solution
|
- The root mean square velocity of one mole of a monoatomic gas having m...
Text Solution
|
- The ratio of most probable velocity to that of average velocity is
Text Solution
|
- Which one of the following statement is not true about the effect of a...
Text Solution
|
- The rms velocity molecules of a gas of density 4 kg m^(-3) and pressur...
Text Solution
|
- Dominance of strong repulsive forces among the molecules of the gas (Z...
Text Solution
|
- The ratio of van der Waals constants a and b, ((a)/(b)) has the dimen...
Text Solution
|
- The term that corrects for the attractive forces present in a real gas...
Text Solution
|
- The van der waals constant 'a' for different gases are given below : ...
Text Solution
|
- If helium is allowed to expand in vacuum, it liberates heat because
Text Solution
|