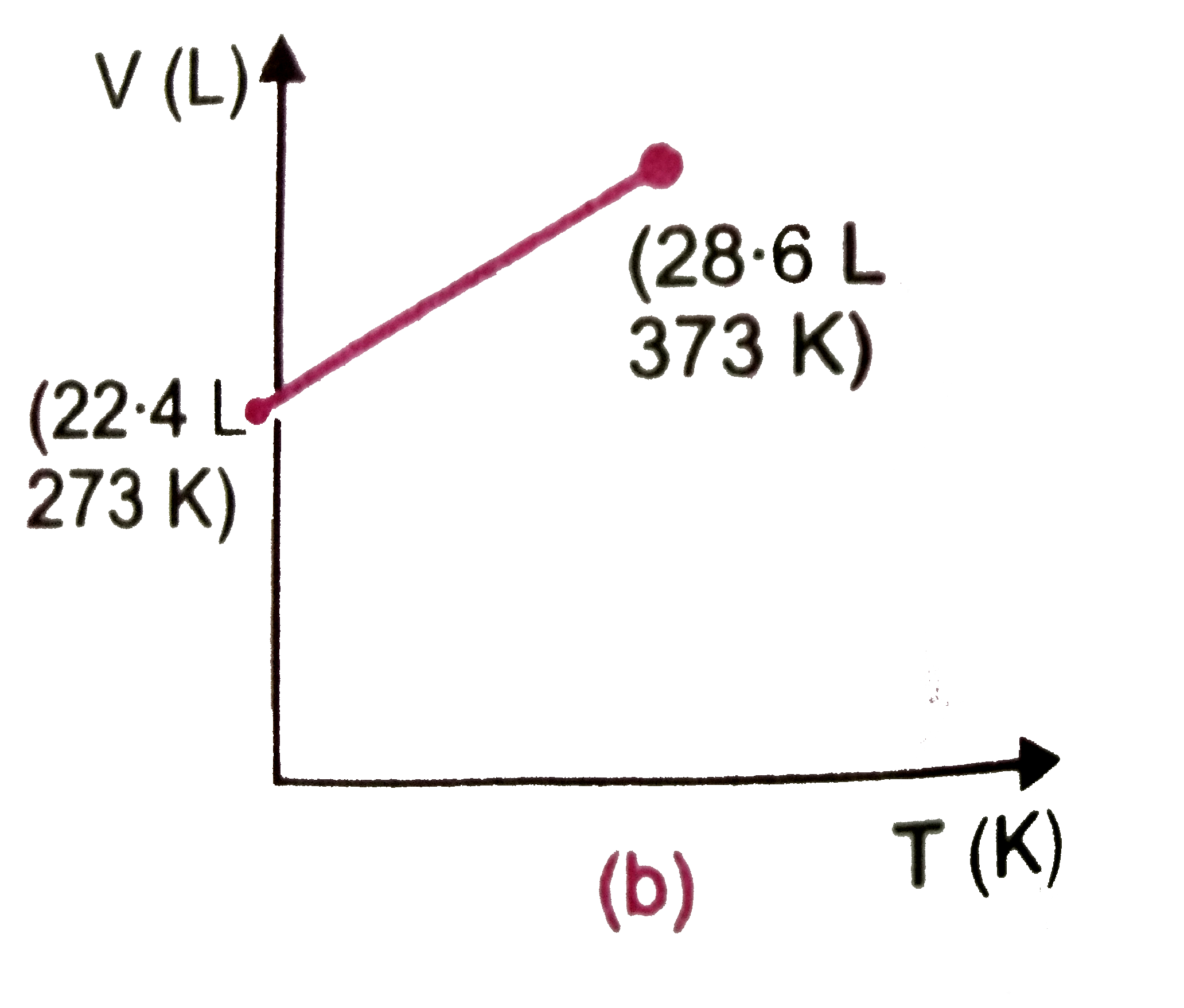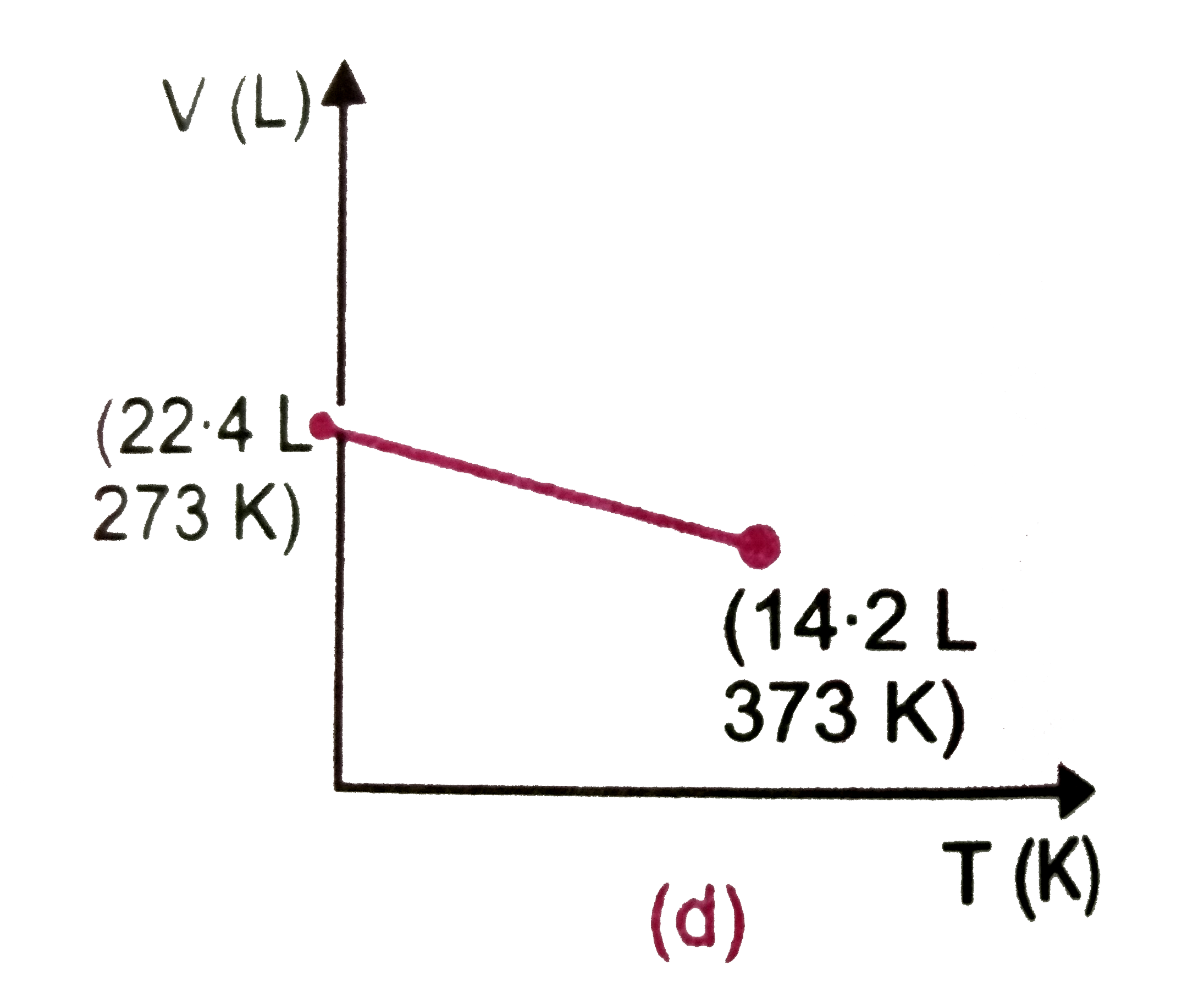A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER : GASES AND LIQUIDES
PRADEEP|Exercise COMPETITION FOCUS (Kinetic theory of gases, kinetic energy and molecular speeds|12 VideosSTATES OF MATTER : GASES AND LIQUIDES
PRADEEP|Exercise COMPETITION FOCUS (Behaviour of real gases and van der Waals equation)|12 VideosSTATES OF MATTER : GASES AND LIQUIDES
PRADEEP|Exercise COMPETITION FOCUS (Differentiating three states of matter and intermolecular forces)|2 VideosSOME p-BLOCK ELEMENTS
PRADEEP|Exercise Competition Focus (JEE( Main and Advanced)/Medical Entrance) VIII. Assertion-Reason Type Questions (Type I)|23 VideosSTATES OF MATTER: SOLID MATTER
PRADEEP|Exercise COMPETITION FOCUS (ASSERTION-REASON)|17 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-STATES OF MATTER : GASES AND LIQUIDES-COMPETITION FOCUS (Measurement of mass, volume, temperature and pressure, gas laws and ideal gas equation
- Average volume available to a molecule in sample of a gas at STP is
Text Solution
|
- Pressure of a mixture of 4 g of O(2) and 2 g H(2) confined in a bulb o...
Text Solution
|
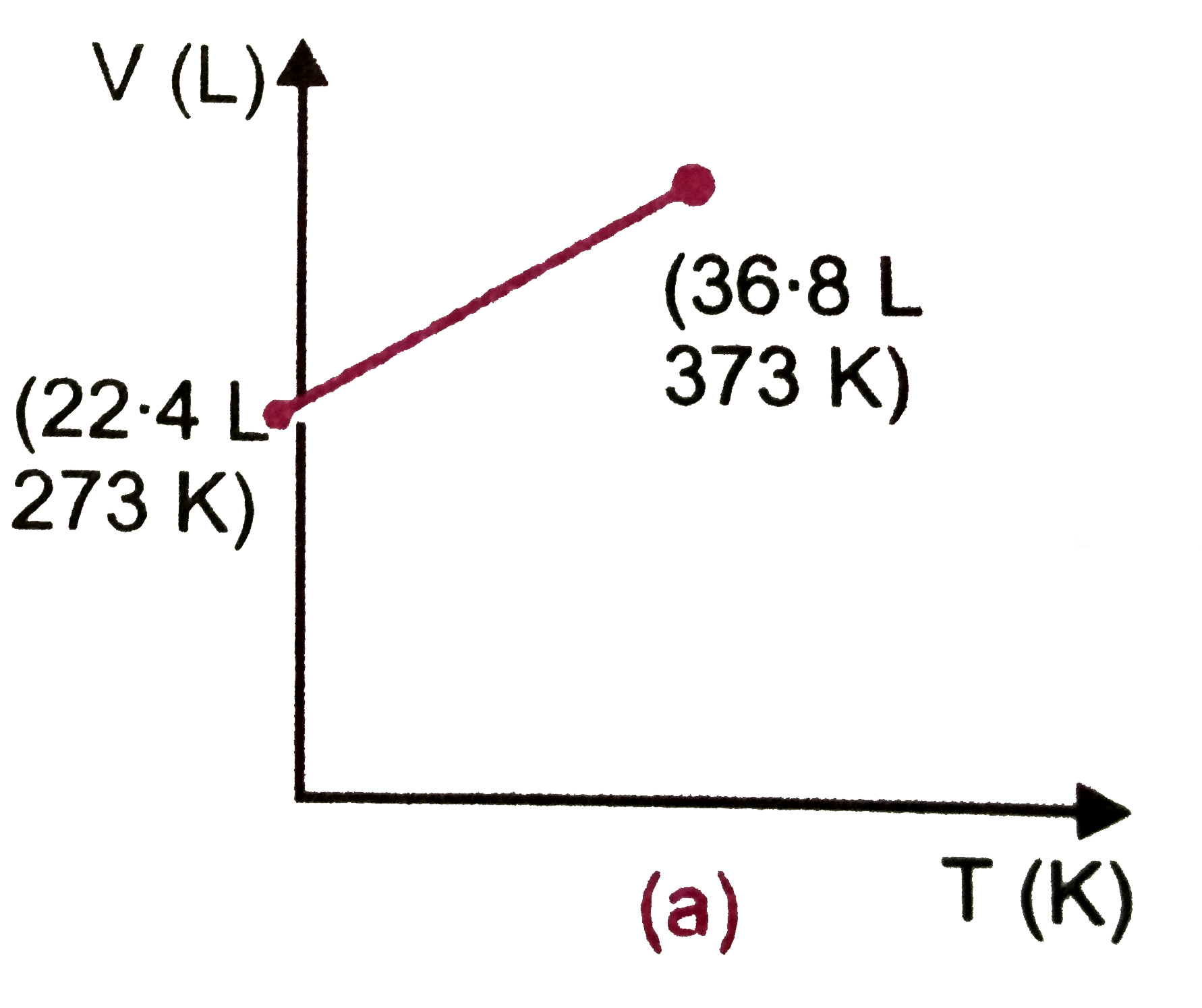
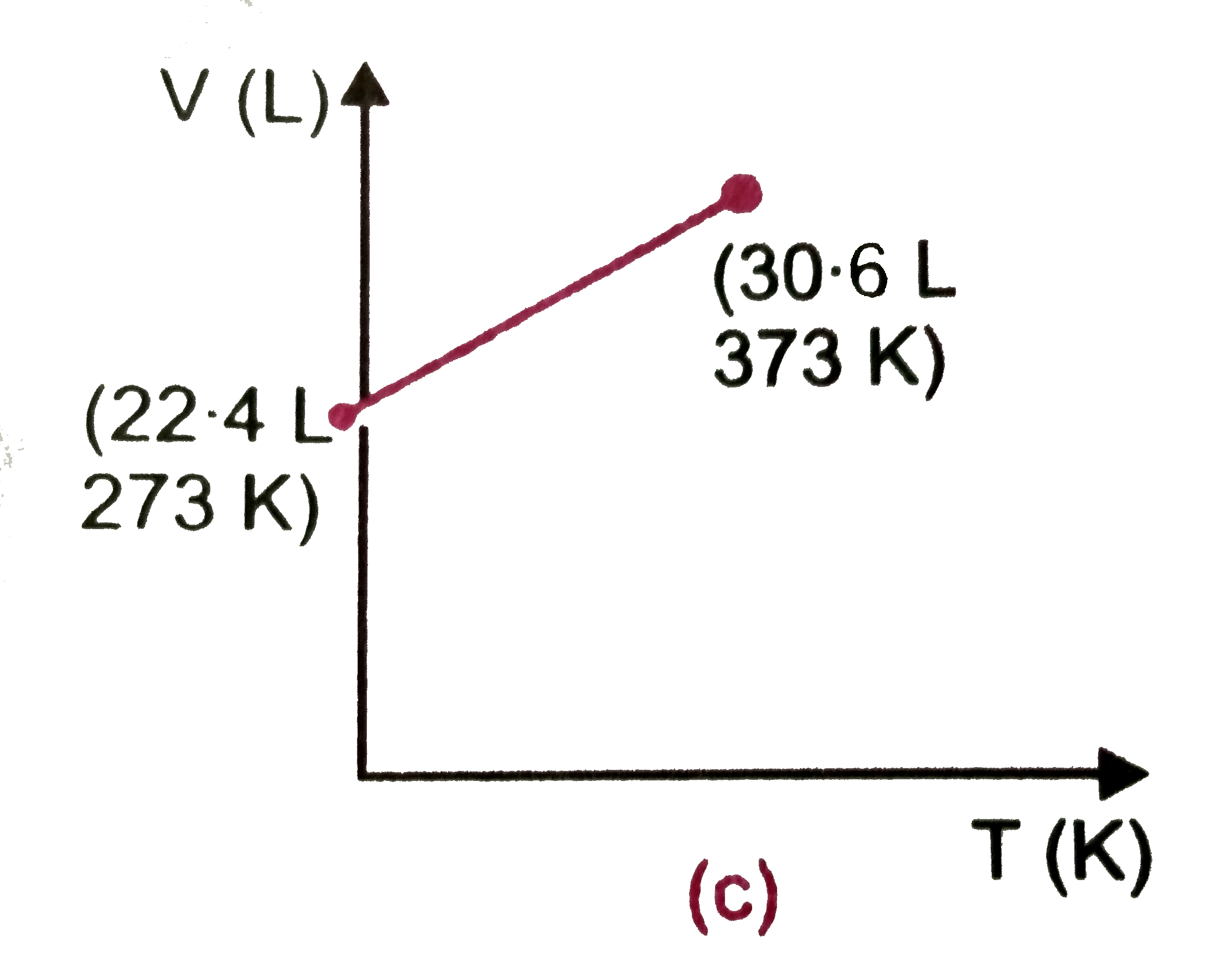
- Which one of the following volume (V)- temperature (T) plots represent...
Text Solution
|
- Containers A and B have same gases. Pressure, volume and temperature o...
Text Solution
|
- A mixture of argon (Ar) and nitrogen (N(2)) has a density of 1.40 g L^...
Text Solution
|
- The density of a gas is 1.964 g dm^(-3) at 273 K and 76 cm Hg. The gas...
Text Solution
|
- If 10^(-4) dm^(3) of water is introduced into a 1.0 dm^(3) flask to 30...
Text Solution
|
- The pressure exerted by 6.0 g of methane gas in a 0.03 m^(3) vessel at...
Text Solution
|
- 2 mole of N(2)O(4) (g) is kept in a closed container at 298 K and 1 at...
Text Solution
|
- What will happen to volume of a bubble of air found under water in a l...
Text Solution
|
- An LPG cylinder containing containing 15 kg butane at 27^(@)C and 10 a...
Text Solution
|
- An evacuated vessel weighs 50 g when empty, 144 g when filled with a l...
Text Solution
|
- 28 g of each of the following gases are taken at 27^(@)C and 600 mm p...
Text Solution
|
- The volume of 0.0168 mol of O(2) obtained by decomposition of KClO(3) ...
Text Solution
|
- Under identical experimental conditions, which one of the following pa...
Text Solution
|
- A bottle of dry ammonia and a bottle of dry hydrogen chloride connecte...
Text Solution
|
- XmL of H(2) gas effuses through a hole in a container is 5 second. The...
Text Solution
|
- A certain gas takes three times as long to effuse out as helium. Its m...
Text Solution
|
- A 4 : 1 mixture of helium and methane contained in a vessel at 10 bar ...
Text Solution
|
- 0.5 mol of H(2), SO(2), and CH(4) is kept in a container. A hole was m...
Text Solution
|