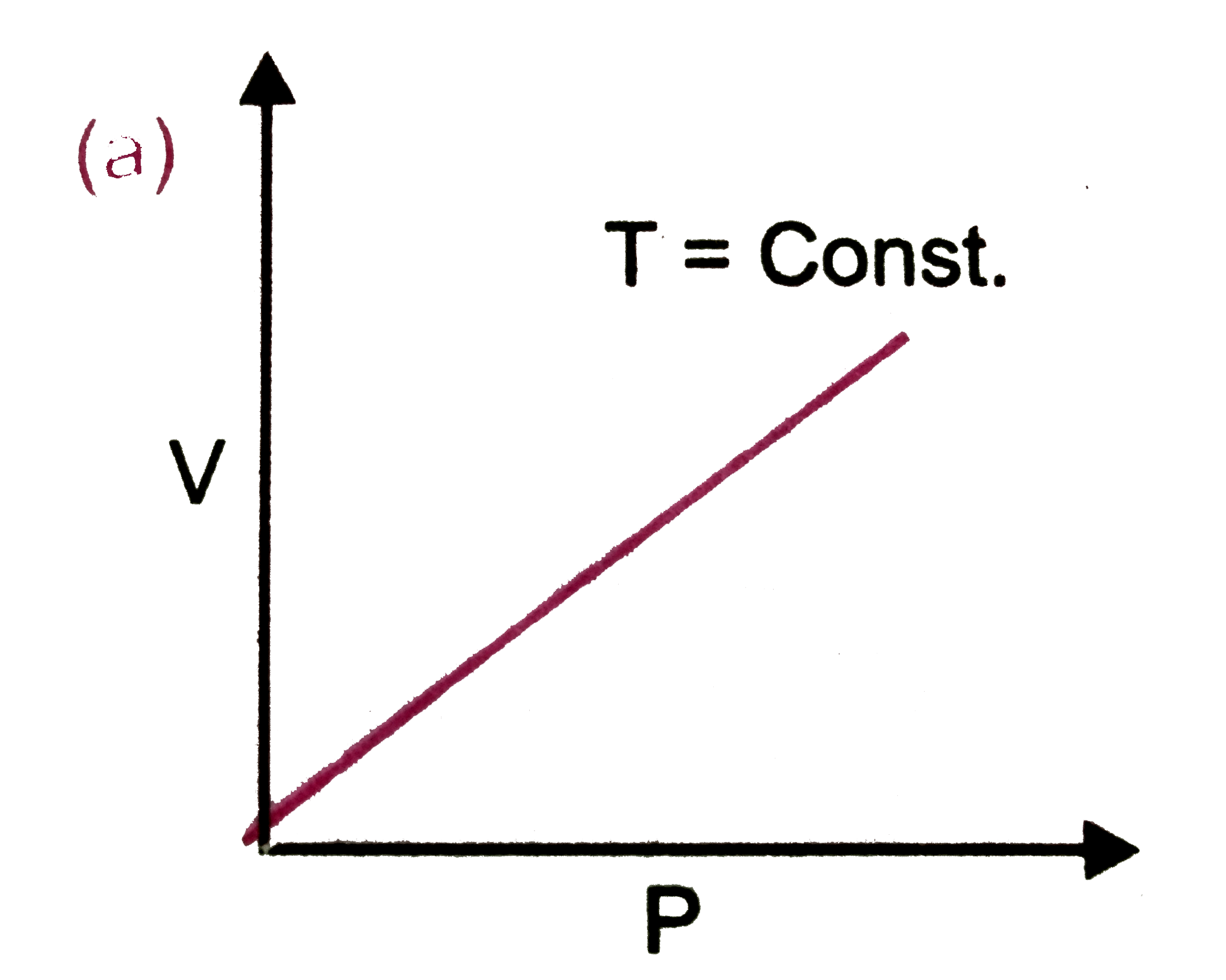
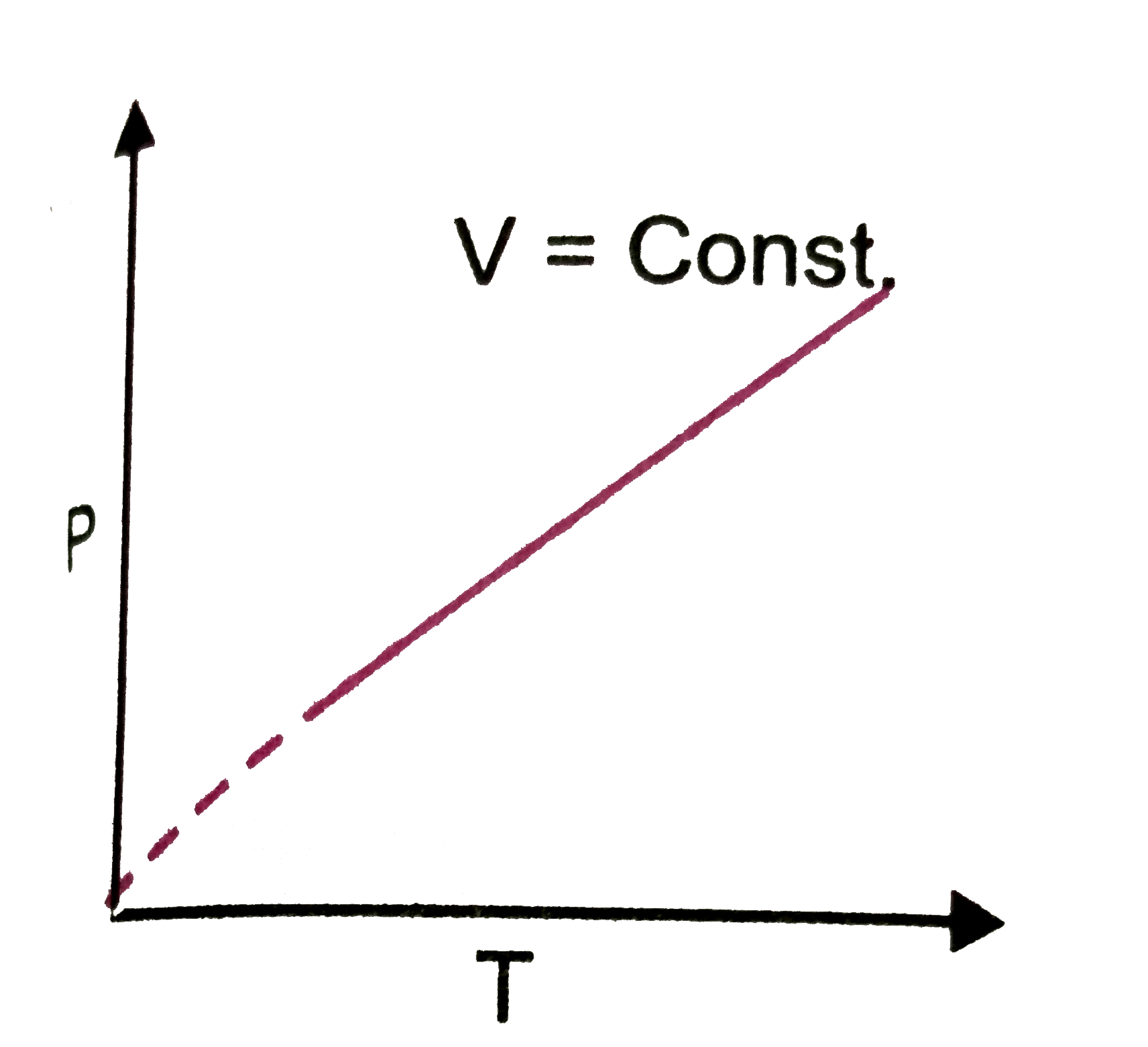
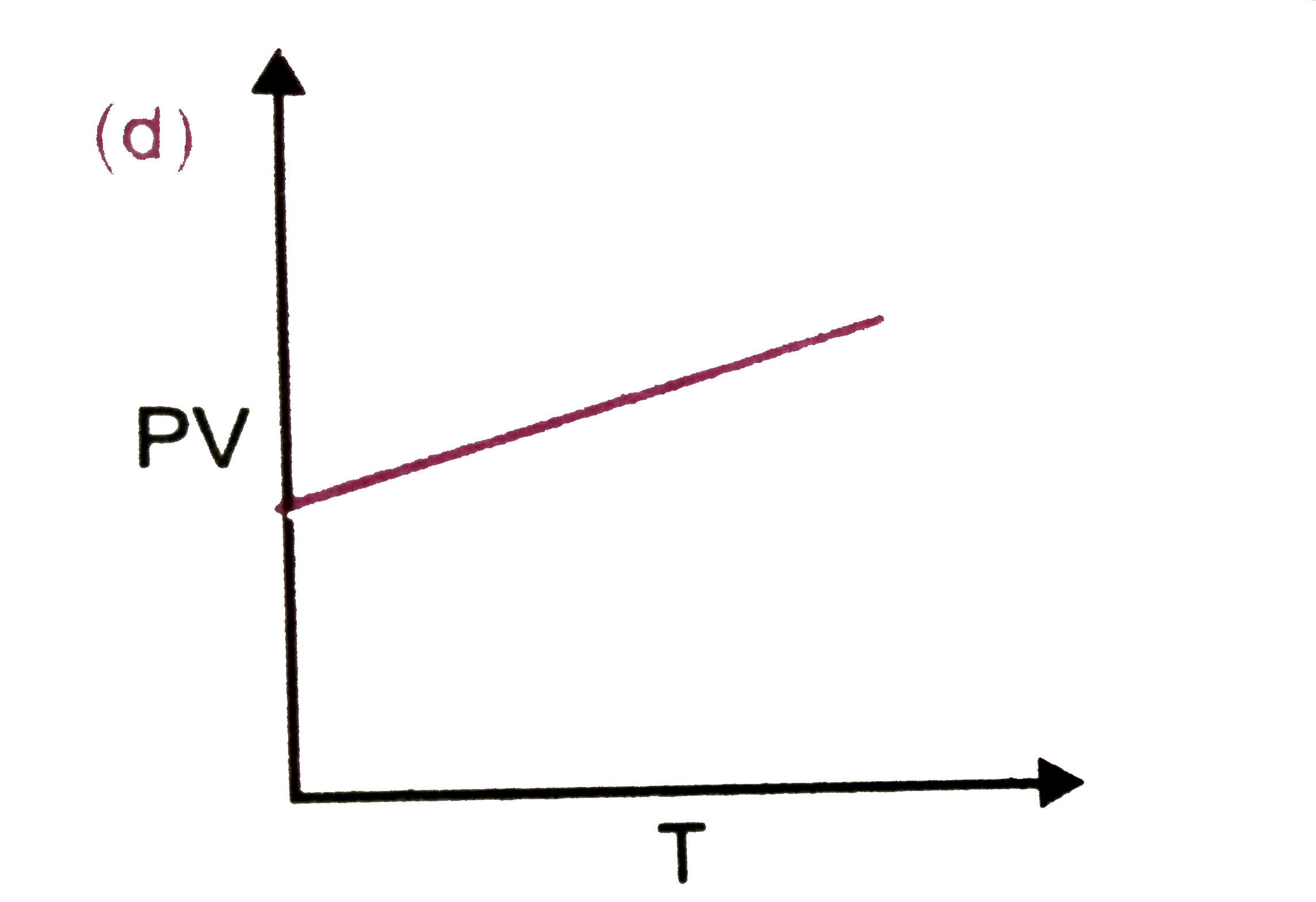
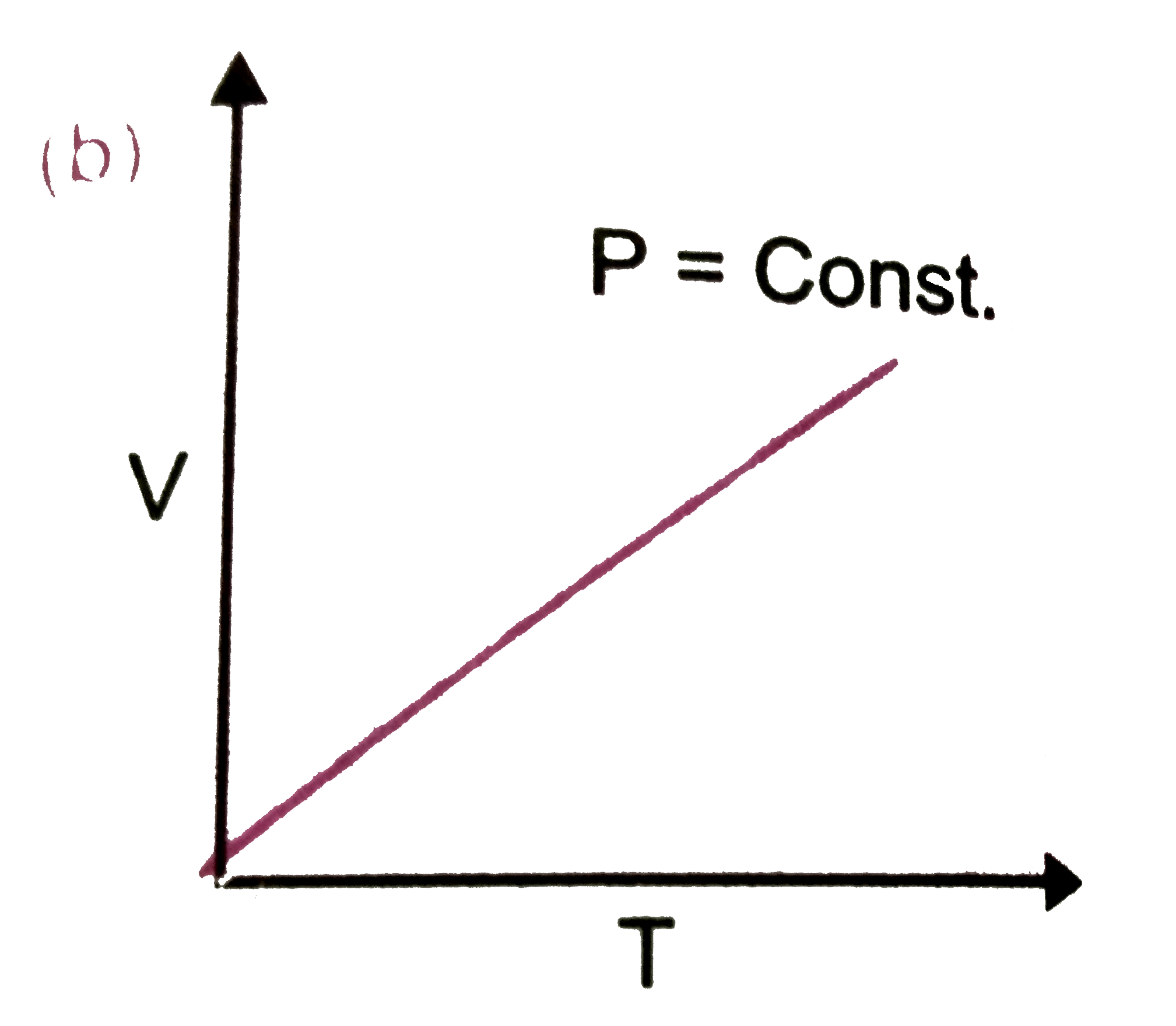A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER : GASES AND LIQUIDES
PRADEEP|Exercise III. Multiple Choice Questions|7 VideosSTATES OF MATTER : GASES AND LIQUIDES
PRADEEP|Exercise IV MATCHING TYPE QUESTIONS|1 VideosSTATES OF MATTER : GASES AND LIQUIDES
PRADEEP|Exercise COMPETITION FOCUS (Miscellaneous)|6 VideosSOME p-BLOCK ELEMENTS
PRADEEP|Exercise Competition Focus (JEE( Main and Advanced)/Medical Entrance) VIII. Assertion-Reason Type Questions (Type I)|23 VideosSTATES OF MATTER: SOLID MATTER
PRADEEP|Exercise COMPETITION FOCUS (ASSERTION-REASON)|17 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-STATES OF MATTER : GASES AND LIQUIDES-II. Multiple Choice Questions
- In the equation PV=RT, the value of R will not depend upon
Text Solution
|
- Boyle's law may be expressed as
Text Solution
|
- Which the following represents the molar volume of the gas correctly
Text Solution
|
- Which of the following plots are correct ?
Text Solution
|
- Which of the following statements are correct ?
Text Solution
|
- A gas described by van der Waals equation .
Text Solution
|
- According to kinetic theory of gases:
Text Solution
|