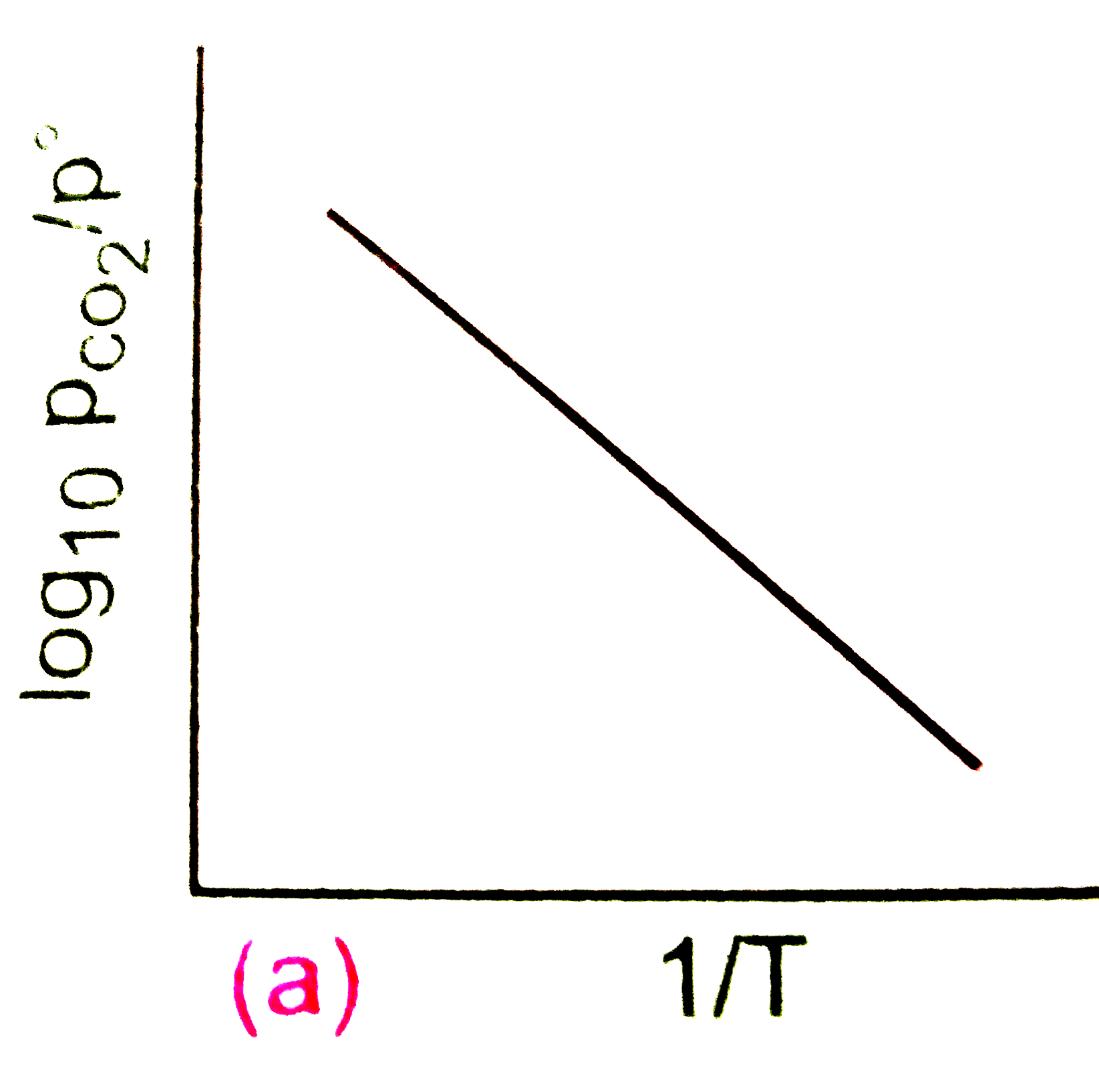
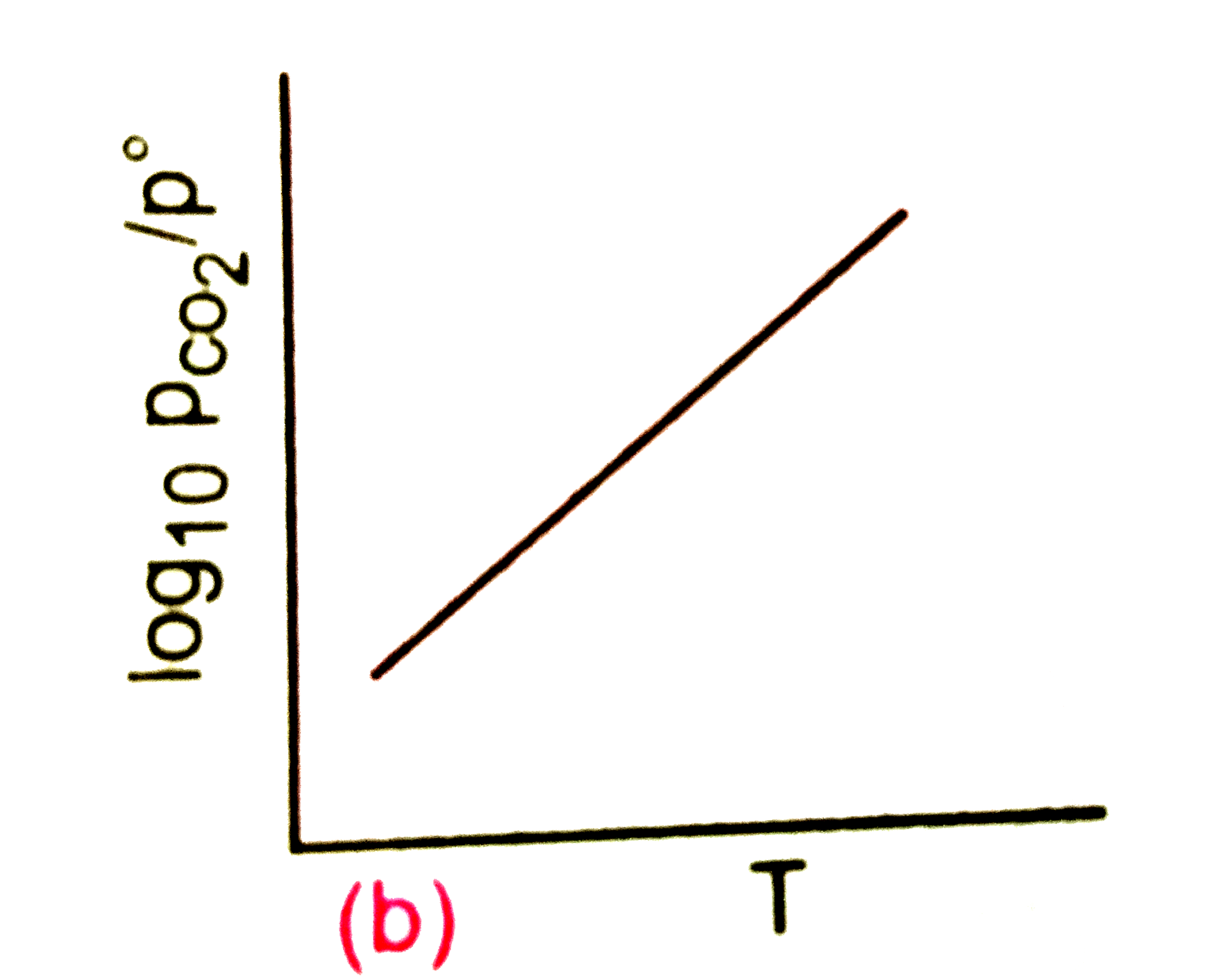
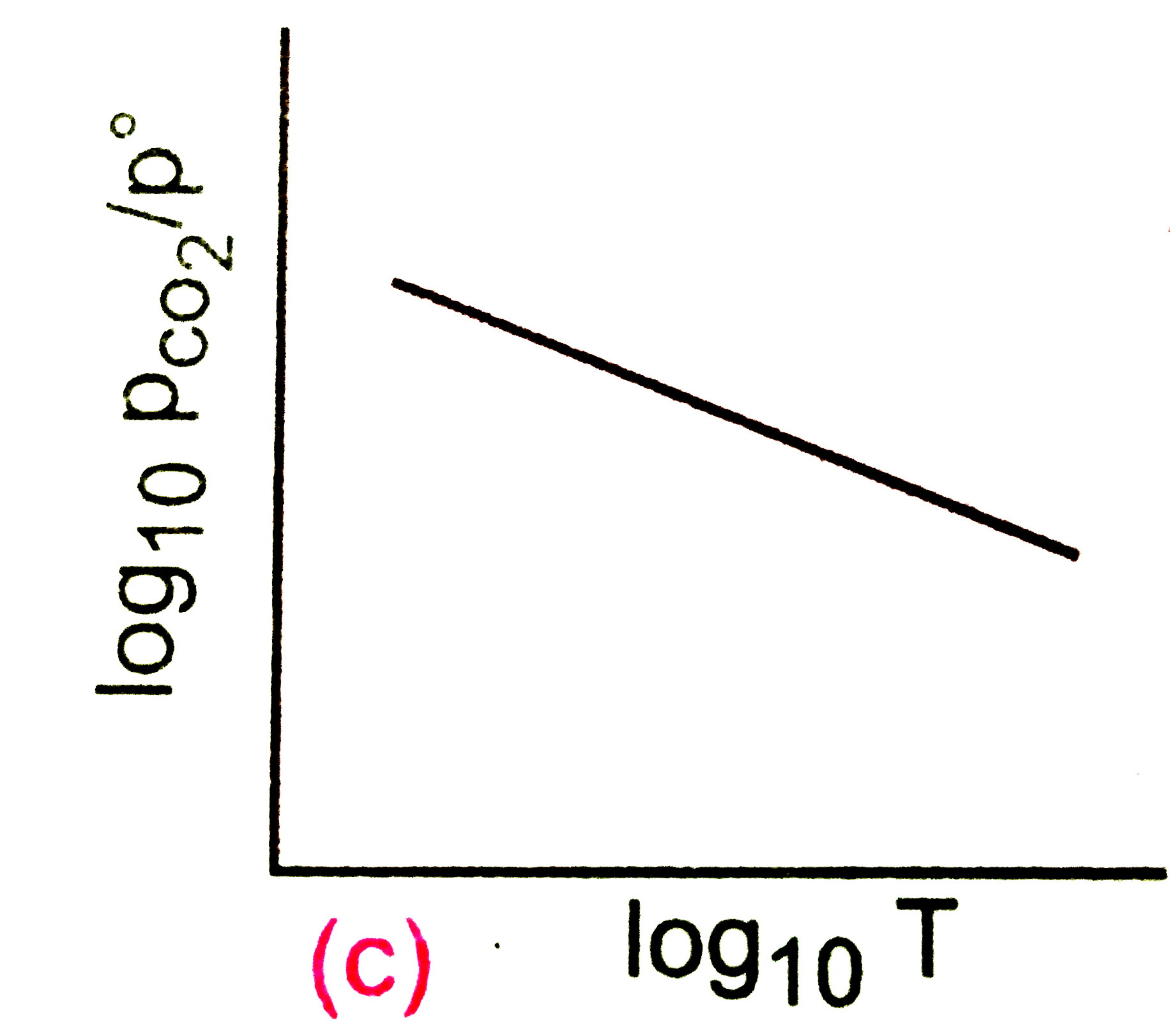
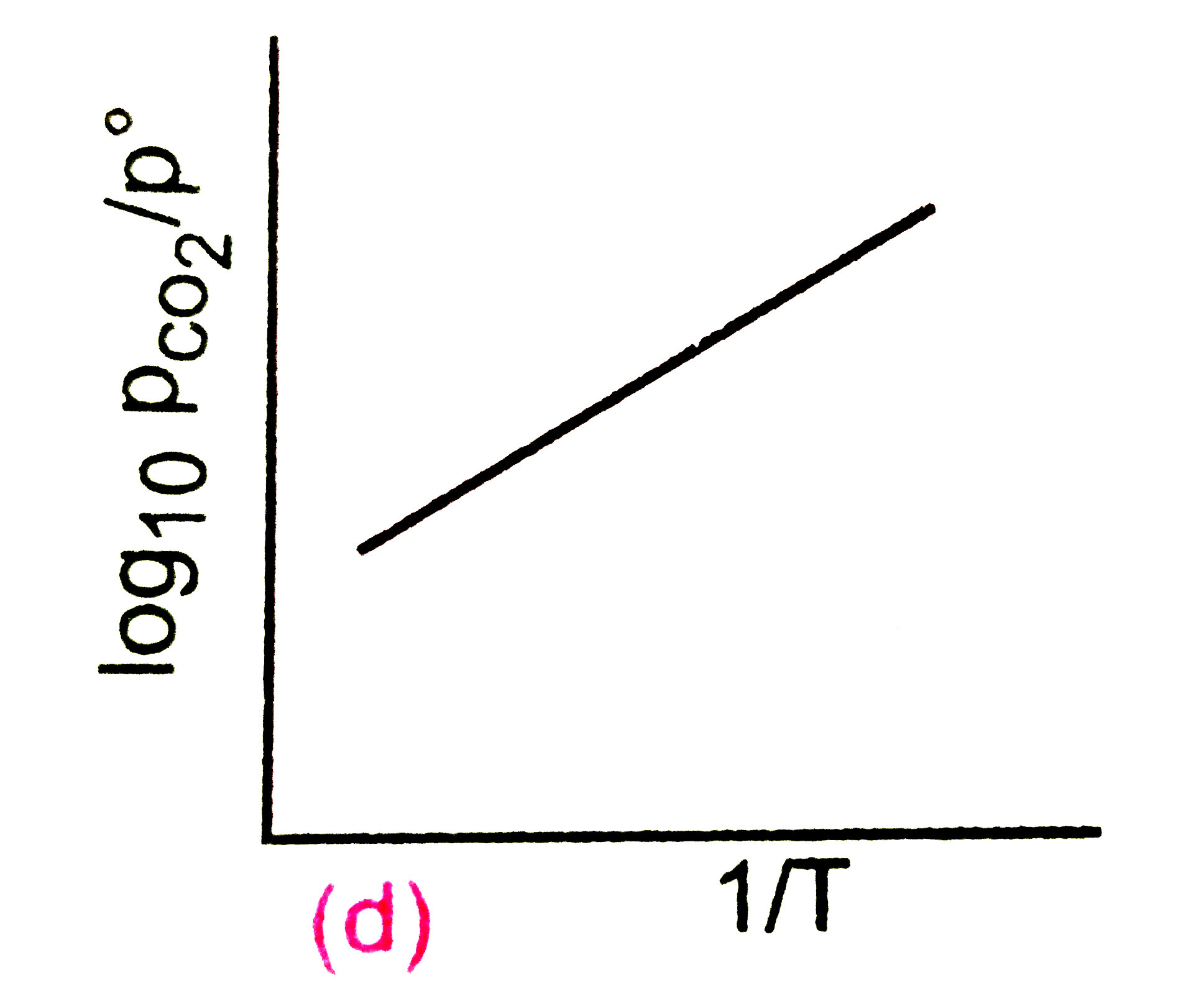
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSES
PRADEEP|Exercise Competition Focus (Jee(Main and advanced)/Medical Entrance) II. MULTIPLE CHOICE QUESTIONS (with one or More One correct answer)|6 VideosEQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSES
PRADEEP|Exercise Competition Focus (Jee(Main and advanced)/Medical Entrance) III. MULTIPLE CHOICE QUESTIONS (Based on the given Passage/Comprehension)|7 VideosEQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSES
PRADEEP|Exercise ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWER/SOLUTIONS (PROBLEMS)|20 VideosEQUILIBRIUM
PRADEEP|Exercise Competition Focus (VIII. Assertion-Reason Type Questions)|16 VideosHYDROCARBONS
PRADEEP|Exercise Competition Focus (JEE(main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS|31 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSES-Competition Focus (Jee(Main and advanced)/Medical Entrance) I. MULTIPLE CHOICE QUESTIONS (with one correct answer)
- Consider the following gaseous equilibria with equilibrium constant K(...
Text Solution
|
- The following equilibria are given by : N(2)+3H(2) hArr 2NH(3), K(1)...
Text Solution
|
- For the chemical equilibrium, CaCO(3)(s) hArr CaO(s)+CO(2)(g) Delt...
Text Solution
|
- A schematic plot of In K(eq) versus inverse of temperature for a rea...
Text Solution
|
- The variation of equilibrium constant ( K) with temperature ( T) was s...
Text Solution
|
- In the preparation of CaO from CaCO(3) using the equilibrium, CaCO(3...
Text Solution
|
- For a given exothermic reaction , K(p) and k'(p) are the equilibrium c...
Text Solution
|
- If the value of equilibrium constant for a particular reaction is1.6xx...
Text Solution
|
- An aqueous solution contains 0.10 M H2S and 0.20 M HCl. If the equilib...
Text Solution
|
- The following equilibrium constants are given : N(2) + 3 H(3) hArr ...
Text Solution
|
- The dissociation constants for acetic acid and HCN at 25^(@)C are 1.5x...
Text Solution
|
- Consider the following reactions in which all the reactants and the pr...
Text Solution
|
- Partial pressure of O(2) in the reaction 1//2 P(2) + 1//2 Q(2) + 1/...
Text Solution
|
- Mercurous chloride , Hg(2)Cl(2), in a saturated solution has the equi...
Text Solution
|
- In a reaction A+2B hArr 2C, 2.0 moles of 'A' 3 moles of 'B' and 2.0 m...
Text Solution
|
- 500 ml vessel contains 1.5 M each of A, B, C and D at equlibrium. If 0...
Text Solution
|
- When two reactants, A and B are mixed to give products C and D, the re...
Text Solution
|
- 9.2 grams of N(2)O(4(g)) is taken in a closed one litre vessel and hea...
Text Solution
|
- Calculate the partial pressure of carbon monoxide from the following d...
Text Solution
|
- The equilibrium: P(4)(g)+6Cl(2)(g) hArr 4PCl(3)(g) is attained by ...
Text Solution
|