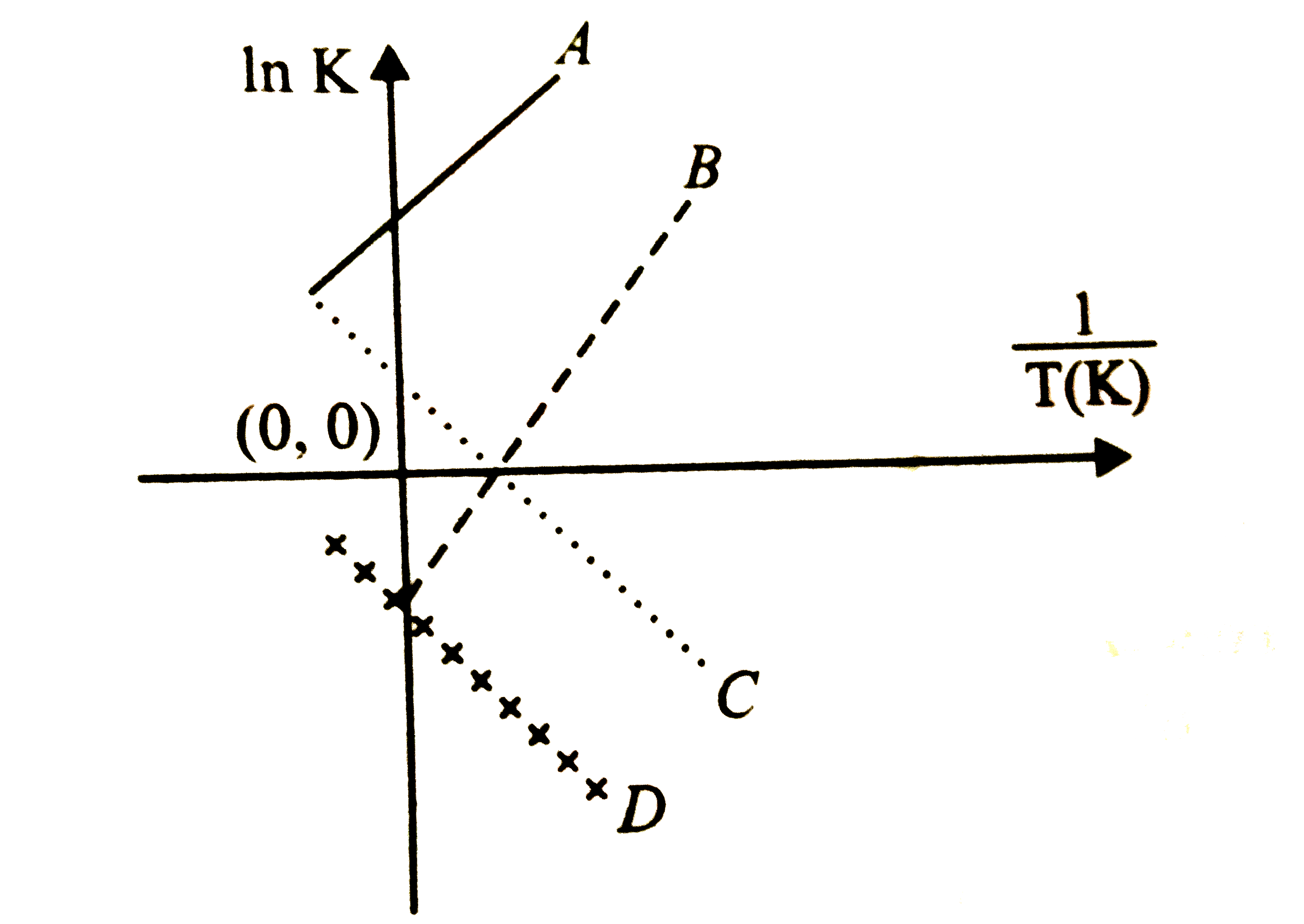
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSES
PRADEEP|Exercise Competition Focus (Jee(Main and advanced)/Medical Entrance) II. MULTIPLE CHOICE QUESTIONS (with one or More One correct answer)|6 VideosEQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSES
PRADEEP|Exercise Competition Focus (Jee(Main and advanced)/Medical Entrance) III. MULTIPLE CHOICE QUESTIONS (Based on the given Passage/Comprehension)|7 VideosEQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSES
PRADEEP|Exercise ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWER/SOLUTIONS (PROBLEMS)|20 VideosEQUILIBRIUM
PRADEEP|Exercise Competition Focus (VIII. Assertion-Reason Type Questions)|16 VideosHYDROCARBONS
PRADEEP|Exercise Competition Focus (JEE(main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS|31 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSES-Competition Focus (Jee(Main and advanced)/Medical Entrance) I. MULTIPLE CHOICE QUESTIONS (with one correct answer)
- Choose the equilibrium that is not influenced by pressure
Text Solution
|
- The reaction , SO(2) + Cl(2) hArr SO(2)Cl(2) is exothermic and reversi...
Text Solution
|
- Consider the following equilibrium in a closed container N(2)O(4)(g)...
Text Solution
|
- Given reaction is 2X((gas)) + Y((gas))hArr2Z((gas)) + 80 Kcal Which ...
Text Solution
|
- The following two reactions: i. PCl(5)(g) hArr PCl(3)(g)+Cl(2)(g) ...
Text Solution
|
- At equilibrium of the reaction 2X(g)+Y(g) hArr X(2)Y(g) the number...
Text Solution
|
- To an equilibrium mixture of 2SO(2) (g) + O(2) (g) hArr 2 SO(3) (g)...
Text Solution
|
- The equilibrium of the reaction N(2)(g)+3H(2)(g) hArr 2NH(3)(g) will b...
Text Solution
|
- The % yield of ammonia as a function as a function of time in the reac...
Text Solution
|
- In which one of the following the increase of presure favours the back...
Text Solution
|
- Consider the reaction equilibrium underset (("Greater volume "))"I...
Text Solution
|
- Which one of the following condition will favour maximum formation of ...
Text Solution
|
- A mixture of NO(2) and N(2)O(4) has a vapor density of 38.3 at 300 K. ...
Text Solution
|
- Ammonium carbamate when heated to 200^(@) C gives a mixture of NH(3) ...
Text Solution
|
- The vapour density of fully dissociated NH(4)Cl would be
Text Solution
|
- N(2)O(4) is 10% dissociated at a total pressure P(1) and 20% dissocia...
Text Solution
|
- At equilibrium of the reaction , N(2)O(4)(g) hArr 2NO(2)(g) the ob...
Text Solution
|
- The values of K(p(1)) and K(p(2)) for the reactions X hArr Y+Z ….(i...
Text Solution
|
- 3 moles of A and 4 moles of B are mixed together and allowed to come ...
Text Solution
|
- Which of the following lines correctly show the temperature dependence...
Text Solution
|