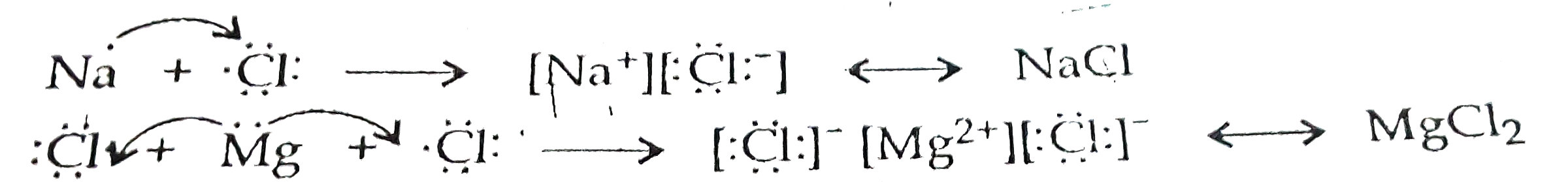Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- (a) Show the formation of magnesium chloride and sodium chloride by tr...
Text Solution
|
- (a) Show the formation of magnesium chloride and sodium chloride by tr...
Text Solution
|
- क्या आयनिक यौगिक ठोस अवस्था में भी विद्युत के चालक होते है ?
Text Solution
|
- a. Explain the formation of Calcium Chloride with the help of electron...
Text Solution
|
- क्या सोडियम क्लोराइड ठोस अवस्था में विद्युत् का चालन कर सकता है।
Text Solution
|
- सोडियम क्लोराइड की क्रिस्टलीय संरचना Na^(+) व Cl^(-) आयनों से निर्मि...
Text Solution
|
- (i) Write the electron-dot structures for sodium, oxygen and magnesium...
Text Solution
|
- (a) Show the formation of Na2O by transfer of electrons between the co...
Text Solution
|
- Define ionic compounds. Ionic compounds conduct electricity only in th...
Text Solution
|