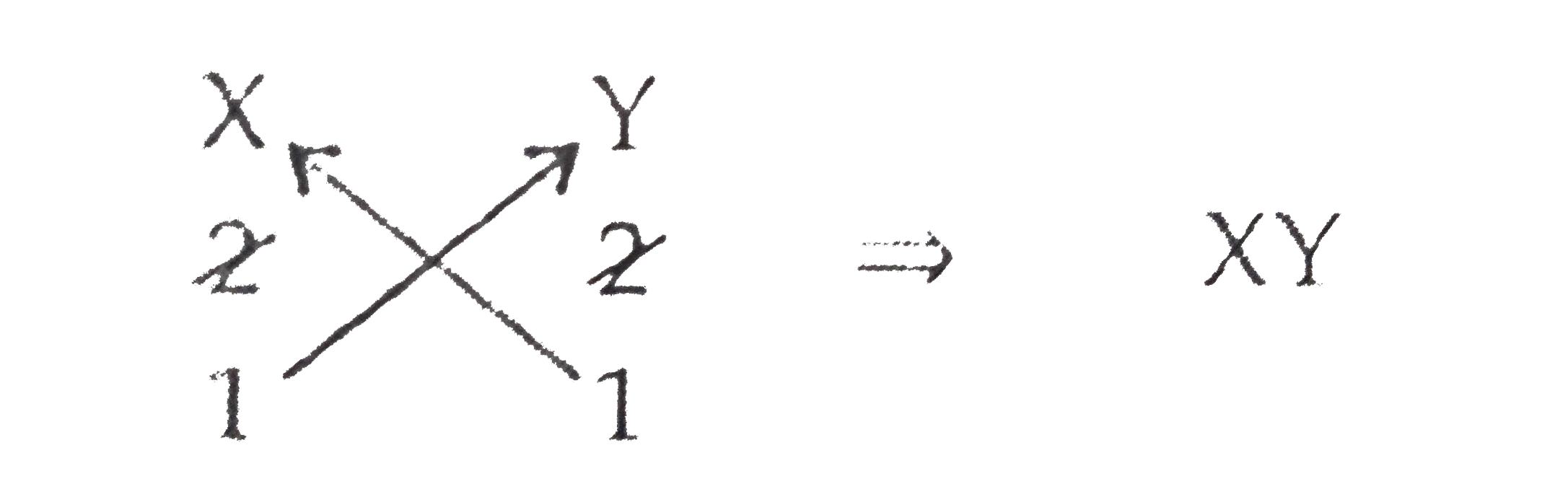(i) Oxides of `1^(st)` group element (Let it be A):
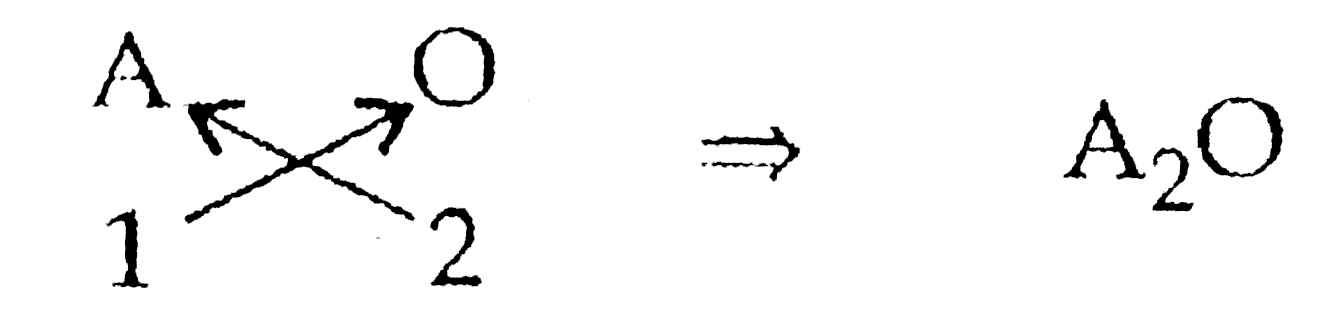
`1^(st)` group element: A Valency : +1
Oxides: O Valency: -2
`therefore` Chemical Formula:
(ii) Halides of the elements of group 13(Let it be M):
Let element of group 13: M Valency : +3
Halide: X Valency: -1
`therefore` Chemical formula:
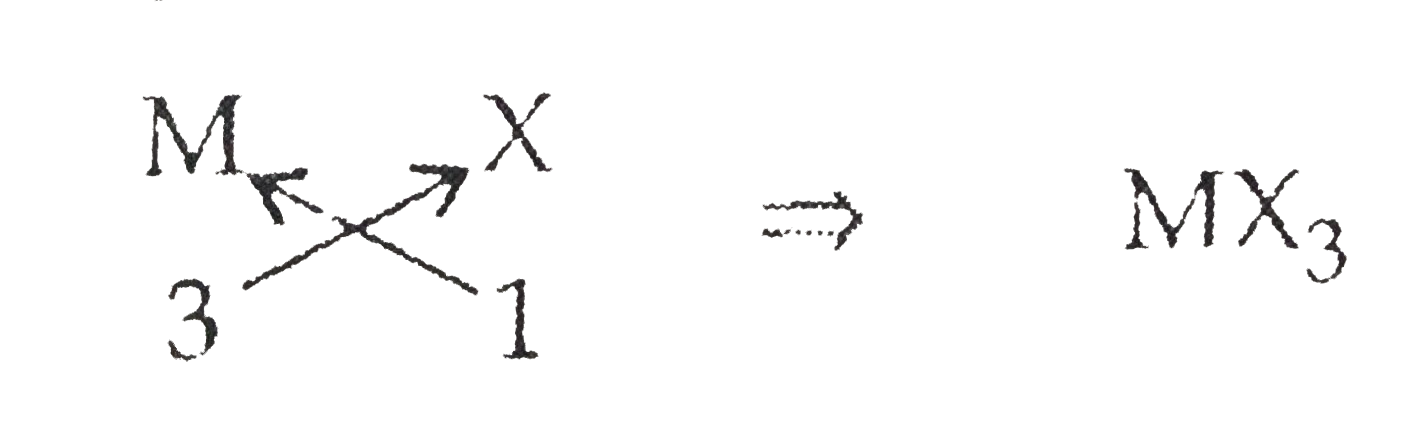
(iii) Compounds of element of group 2 combines with an element of group 16 (Y):
Element of group 2: X Valency: +2
Element of group 16: Y Valency: -2
`therefore` Chemical Formula: