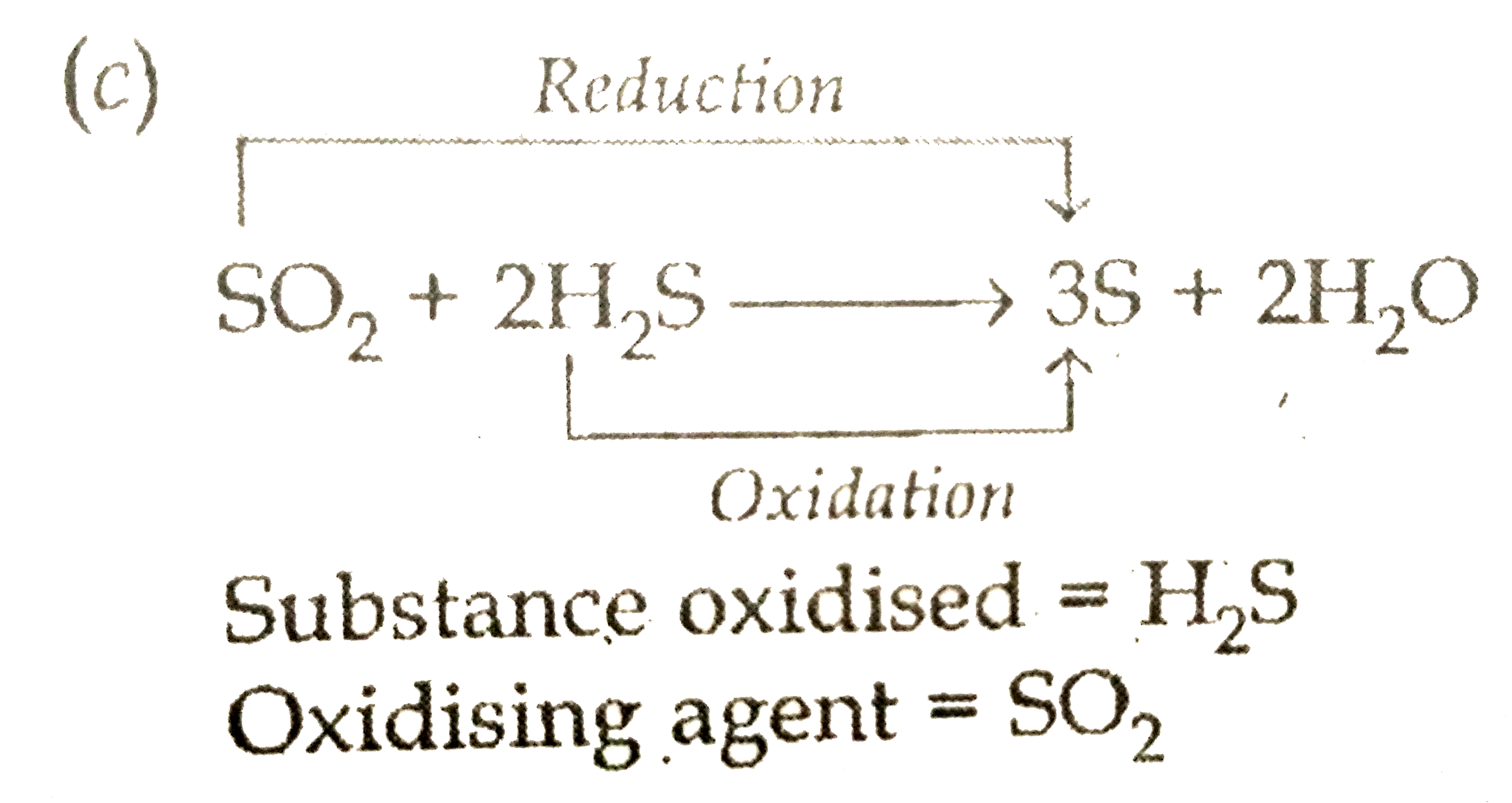
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Name the substance oxidised and the substance reduced, and also identi...
Text Solution
|
- Name the substance oxidised and the substance reduced, and also identi...
Text Solution
|
- In the reaction, SO(2)+2H(2)S to 3S+2H(2)O , the substance oxidised is
Text Solution
|
- From the following equations, identify the substances oxidzed and redu...
Text Solution
|
- Name the oxidising and reducing agent in the following reaction: 2H(...
Text Solution
|
- Identify the substances oxidised, substance reduced, oxidising agent a...
Text Solution
|
- Name the reduching agent in the following reaction. 3MnO(2)+4Al to 2...
Text Solution
|
- Identify the reducing agent and oxidizing agent in the following react...
Text Solution
|
- Name the substance oxidised and reduced, and also identify the oxidisi...
Text Solution
|