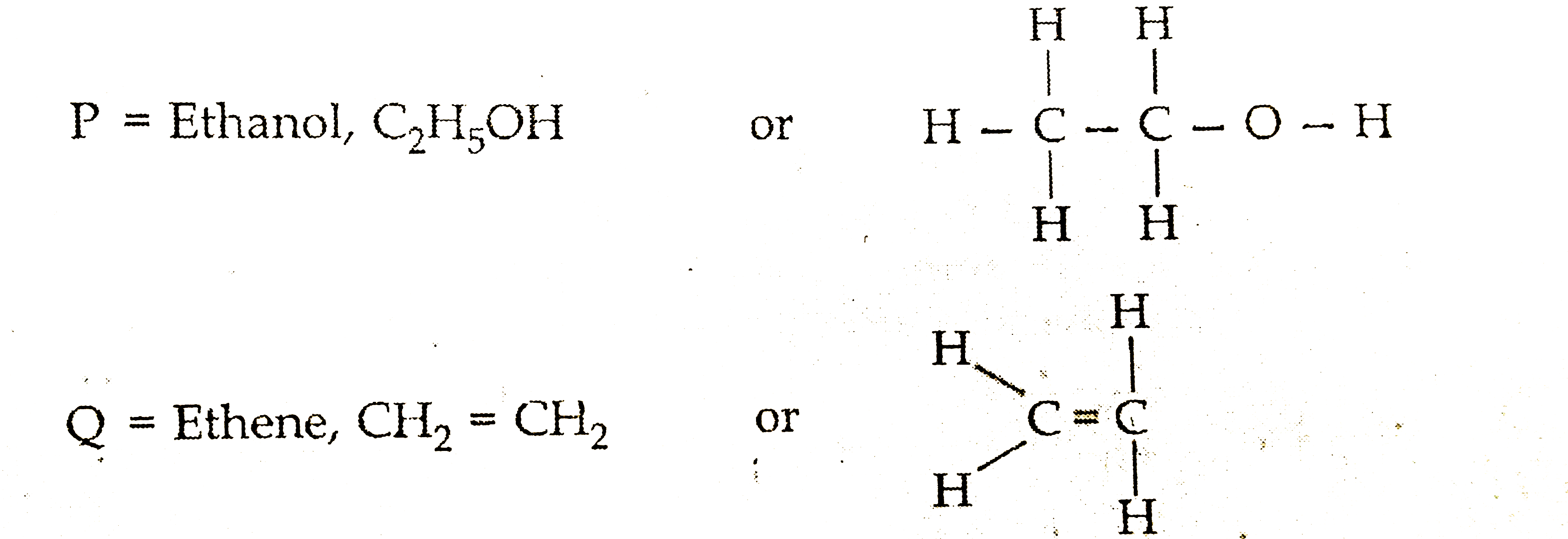
`R = "Ethane", C_(2)H_(6)" "or" "H-underset(H)underset(|)overset(H)overset(|)C-underset(H)underset(|)overset(H)overset(|)C-H`
Explanation:
When ethanol is heated with excess of conc. `H_(2)SO(4)`, it gets dehydrated to form ethene (an unsaturated hydrocabon)
`CH_(3)-underset((P))underset("Ethanol")(CH_(2))-OH underset(170^(@)C) overset(Conce. H_(2)SO_(4))rarr underset((Q))underset("Ethene")(CH_(2))=CH_(2)+H_(2)O`
When ethene is heated with hydrogen in the presence of nickel catalyst it forms ethane (a saturated hydrocarbon)
`CH_(2)=CH_(2)+H_(2) underset("Heat")overset("Ni Catalyst")rarr underset((R))underset("Ethane")(CH_(3))-CH_(3)`
`CH_(3)-CH_(3)+(7)/(2)O_(2) rarr 2CO_(2)+3H_(2)O + "Heat" + "Light"`