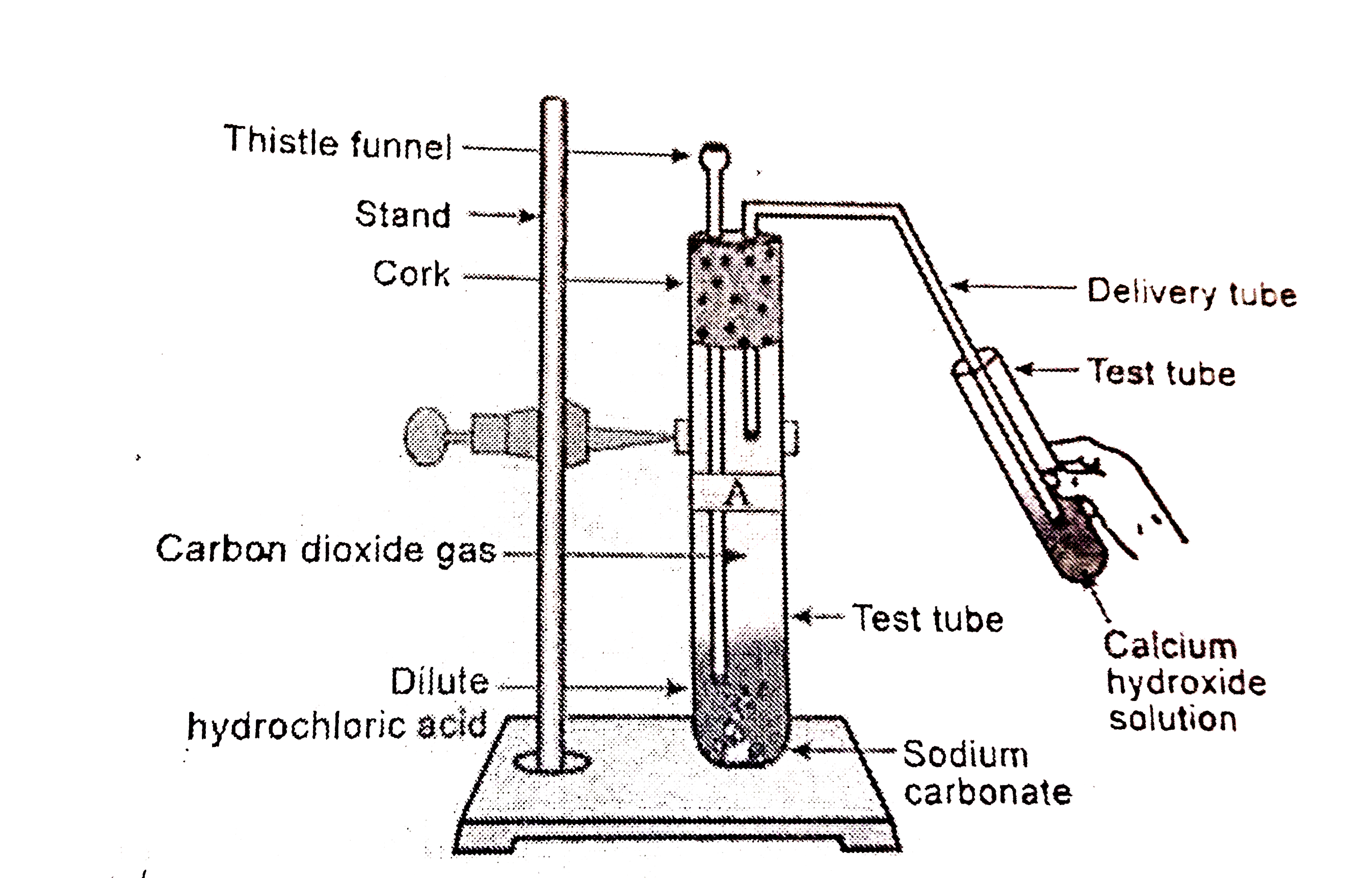
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Write an activity to show the reaction of acids with metal carbonates ...
Text Solution
|
- (a) Describe an activity with diageam to illustrate that the reaction ...
Text Solution
|
- Mineral acids react with metallic carbonate to form its respective met...
Text Solution
|
- Write an activity to show the reaction of acids with metal carbonates ...
Text Solution
|
- धातु कार्बोनेट और धातु हाइड्रोजन कार्बोनेट अम्लों से क्रिया करके क...
Text Solution
|
- Show that the reaction of carbonates and metal hydrogen carbonates wit...
Text Solution
|
- What is the action of acids with carbonates and metal hydrogen carbona...
Text Solution
|
- What is the action of acids with carbonates and metal hydrogen carbona...
Text Solution
|
- కార్బోనేట్ లు మరియు లోహ హైడ్రోజన్ కార్బోనేట్ తో ఆమ్లాల చర్యలను వ్రాయుమ...
Text Solution
|