Hydrocarbons. As these compounds have only atoms of carbon and hydrogen elements in their molecules, so these are called Hydrocarbons.
General formulae:
Alkanes `C_(n) H_(2n+2)`
Alkenes `C_(n)H_(2n)`
Alkynes `C_(n)H_(2n-2)`
Structures of first member of each:
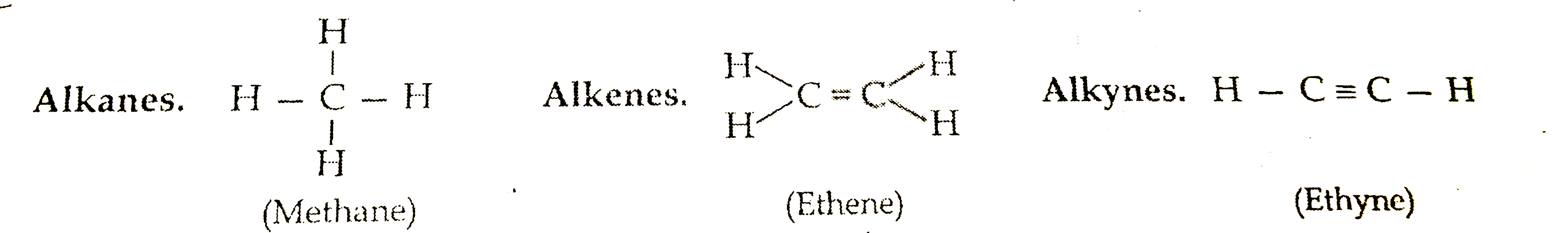
The reaction which convert Alkenes into Alkanes is called Addition Reaction.
`underset("(Ethene)")(H_(2)C=CH_(2)+H_(2)) underset("Heat")overset(Ni) (to) H_(3)Cunderset("Ethane")underset("")(-CH_(3))`
Ni (Nickel) metal acts as catalyst in this reaction.