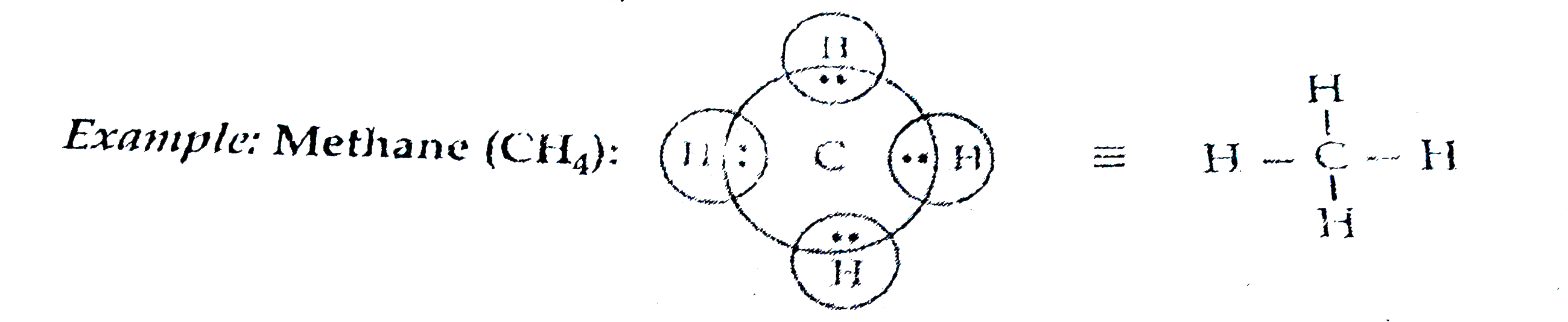
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Carbon has four electrons in its valence shell. Which type of compound...
Text Solution
|
- Carbon has four electrons in its valence shell. Which type of compound...
Text Solution
|
- कार्बन के संयोजी कोष में दो आयुग्मित इलेक्ट्रॉन उपस्थित होते है जबकि इ...
Text Solution
|
- कार्बन ( 2, 4 ) के संयोजकता - कोश में चार इलेक्ट्रॉन होते है। कार्बनि...
Text Solution
|
- In methane molecules , central carbon atom has electrons in its ...
Text Solution
|
- (a)ऐसा यौगिक बताए।(i) जिसमें कार्बन की चारों संयोजकताऍ। एकल संयोजी ...
Text Solution
|
- In which of the following compound has only one type of hybridised car...
Text Solution
|
- Elements forming ionic compounds attain noble gas electronic configura...
Text Solution
|
- Carbon forms ionic compounds by losing four electrons.
Text Solution
|