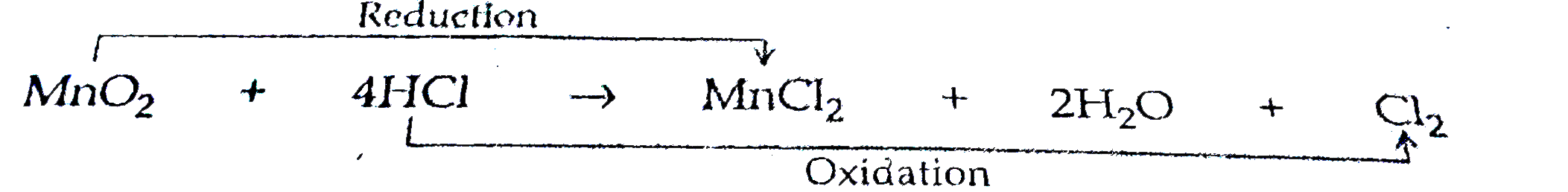Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the reaction : MnO(2) + 4HCI to MnCl(2) + 2H(2)O + Cl(2) (a) N...
Text Solution
|
- Define (i) Oxidation, and (ii) reduction. Identifiy the substances o...
Text Solution
|
- In the reaction MnO(2) + 4HCl to MnCl(2) + 2H(2)O + Cl(2) , identify w...
Text Solution
|
- What is redox reaction ? Identify the substance oxidised and the subst...
Text Solution
|
- ऑक्सीकरण तथा अपचायक से क्या तातपर्य है ? निम्नलिखित अभिक्रियाओं में ऑक...
Text Solution
|
- निम्नलिखित अभिक्रियाओं में ऑक्सीकारक तथा अपचायक बताइए - (i) 2FeCl(3)...
Text Solution
|
- In the reaction : MnO(2) + 4HCI to MnCl(2) + 2H(2)O + Cl(2) (a) N...
Text Solution
|
- MnO(2)+4HCl rarr MnCl(2)+2H(2)O+Cl(2) In the above equation, name th...
Text Solution
|
- Identify the substances oxidised, substance reduced, oxidising agent a...
Text Solution
|