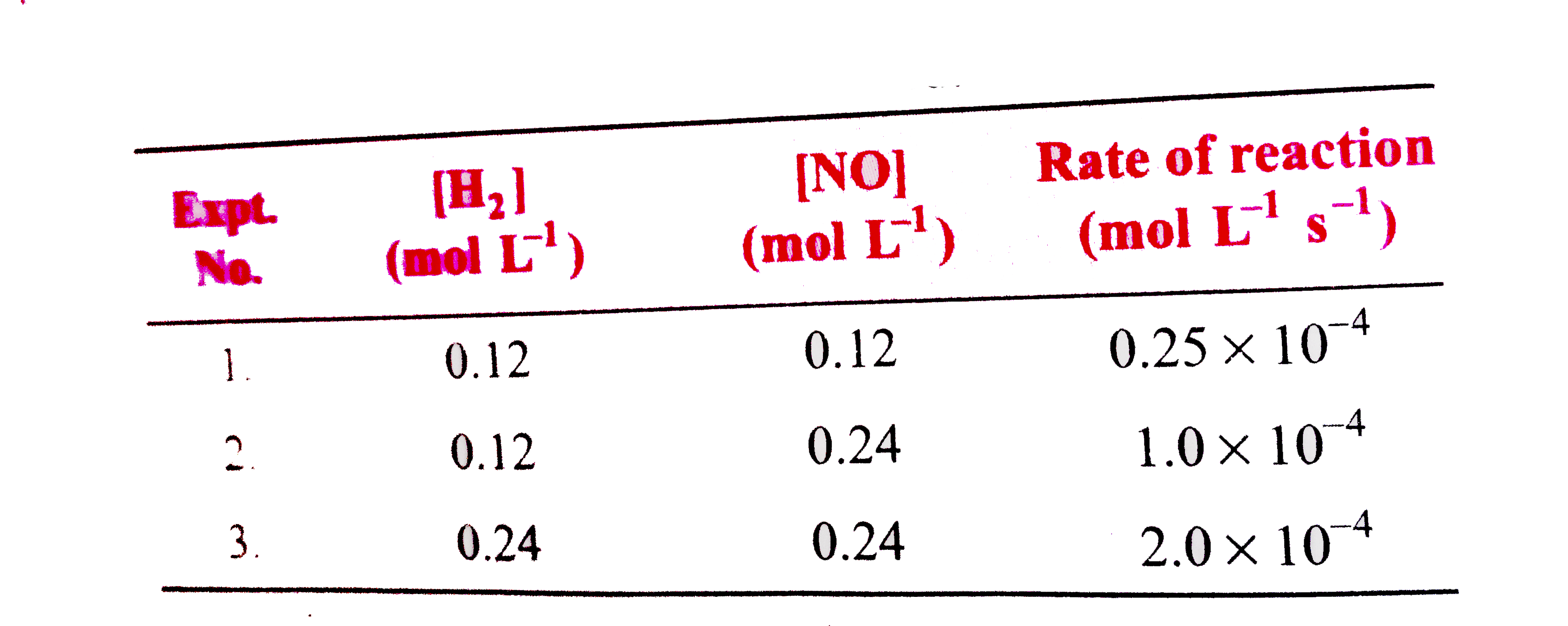Similar Questions
Explore conceptually related problems
Recommended Questions
- For the reaction, 2NO+2H(2)rarrN(2)+2H(2)O, the following kinetic ...
Text Solution
|
- 2NO+2H(2)rarr N(2)+2H(2)O. The experimental rate law for above reactio...
Text Solution
|
- Consider the following reaction , 2NO(g) + 2H(2)(g) to N(2)(g) + 2H(2)...
Text Solution
|
- For the reaction, 2NO+2H(2)rarrN(2)+2H(2)O , the following kinetic dat...
Text Solution
|
- Consider the reaction : 2H(2)(g)+2NO(g)rarrN(2)(g)+2H(2)(g) The rate l...
Text Solution
|
- For the reaction 2H(2)(g)+2NO(g) rarr N(2)(g)+2H(2)O(g) the observed r...
Text Solution
|
- The unit of rate constant for the reaction, 2H(2) + 2NO to 2H(2)O + N(...
Text Solution
|
- The equation and rate law for the gas phase reaction between NO and H(...
Text Solution
|
- For the reaction 2H(2)(g)+2NO(g)rarrN(2)(g)+2H(2)O(g) Rate = k[H(2)][N...
Text Solution
|