Similar Questions
Explore conceptually related problems
Recommended Questions
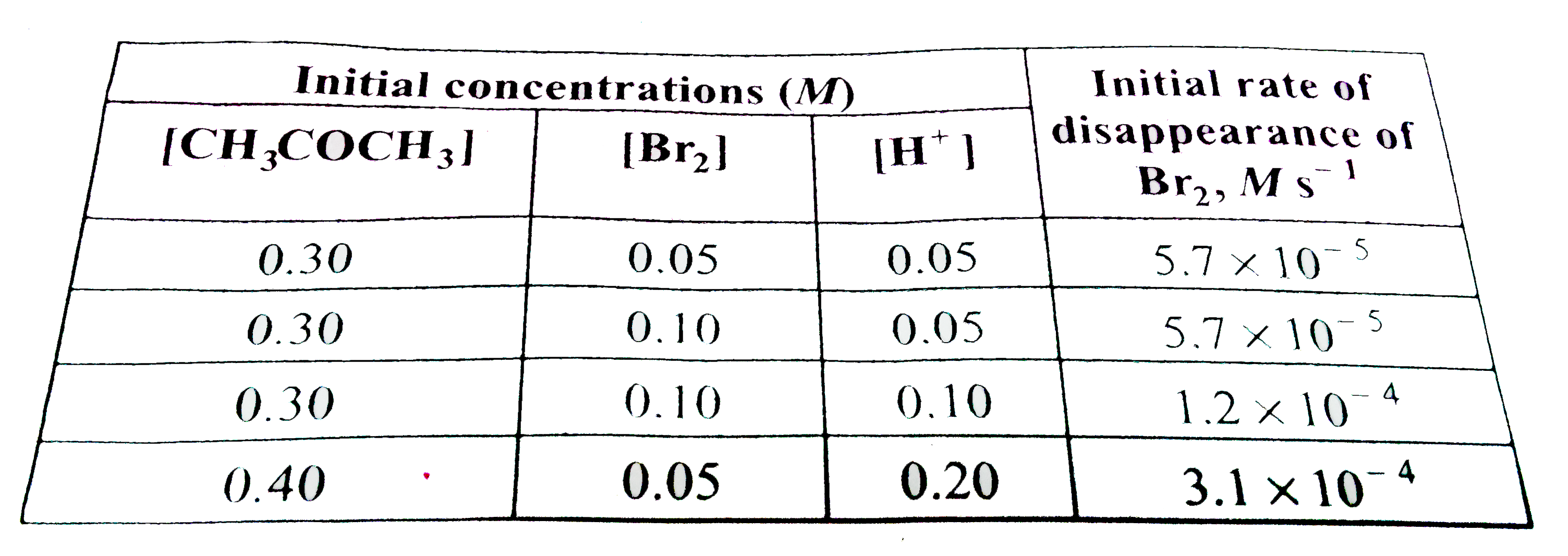
- The bromination of acetone that occurs in acid solution is represente...
Text Solution
|
- The bromination of acetone that occurs in acid solution is represented...
Text Solution
|
- The bromination of acetone which occurs in acid solution is represente...
Text Solution
|
- The bromination of acetone that occurs in acid solution is represente...
Text Solution
|
- The bromination of acetone that occurs in acid solution is represented...
Text Solution
|
- In the reaction BrO(3)^(-) (aq) + 5 Br^(-) (aq) + 6 H^(+) to 3 Br(2) (...
Text Solution
|
- The order of the reaction 5" Br"^(-)(aq)+BrO(3)^(-)(aq)+6" H"^(+)(aq...
Text Solution
|
- The bromination of acetone that occurs in acid solution is represented...
Text Solution
|
- Based on data given below for the electrode potential , reducing power...
Text Solution
|