Similar Questions
Explore conceptually related problems
Recommended Questions
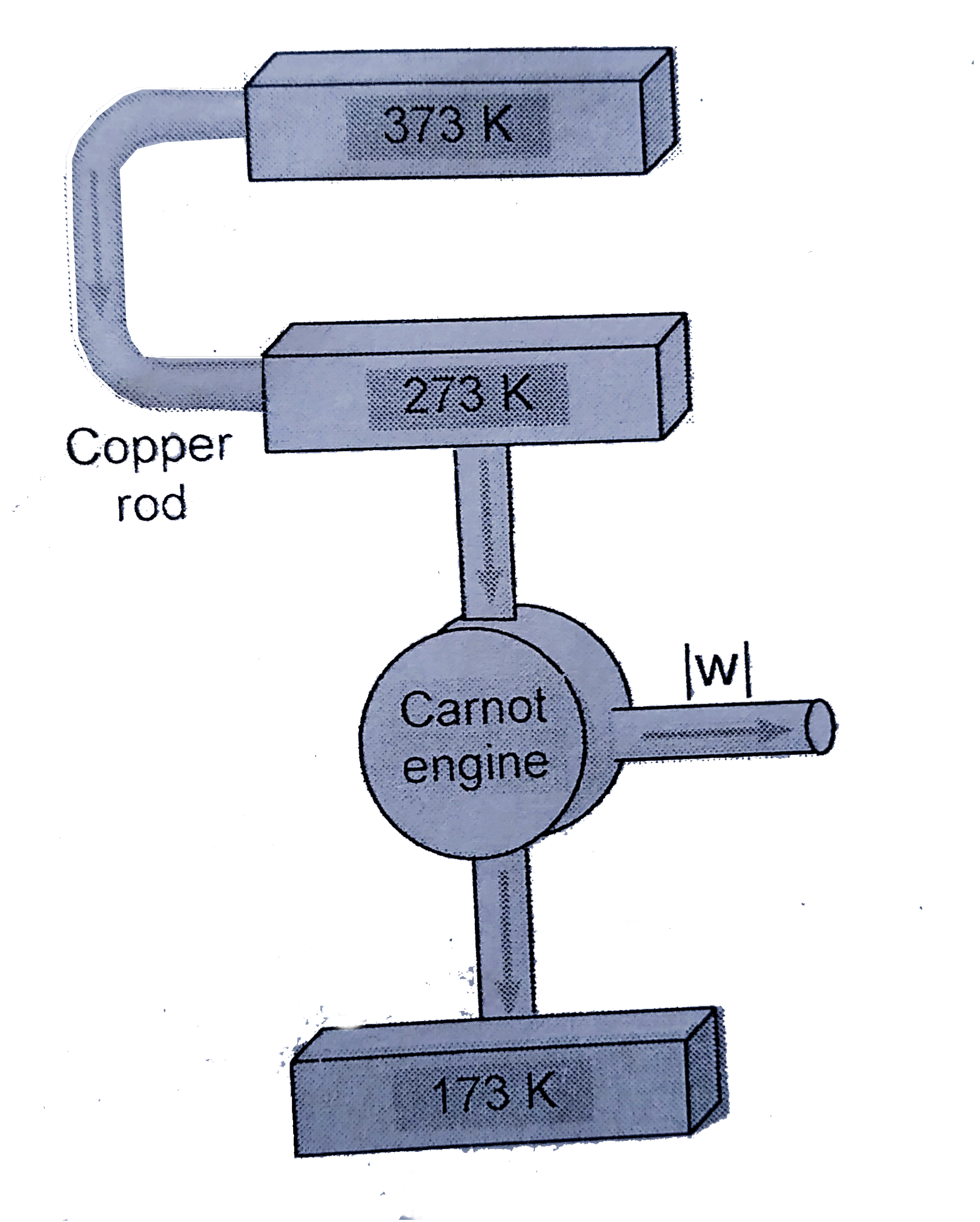
- Heat flows from a reservoir at 373 K to a reservoir at 273 K through a...
Text Solution
|
- Heat flows from a reservoir at 373 K to a reservoir at 273 K through a...
Text Solution
|
- A reversible heat engine A(based on carnot cycle ) absorbs heat fro...
Text Solution
|
- A reversible heat engine A(based on carnot cycle ) absorbs heat fro...
Text Solution
|
- Two Carnot engines A and B are operated in series. The first one, A, r...
Text Solution
|
- A heat of 1200 J is supplied to an engine from a hot reservoir maintai...
Text Solution
|
- A reversible heat engine A (based on carnot cycle) absorbs heat from a...
Text Solution
|
- The hot reservoir for a Carnot engine has a temperature of 890 K, whil...
Text Solution
|
- A Carnot engine receives 2.0 kj of heat from a reservoir at 500 K, doe...
Text Solution
|