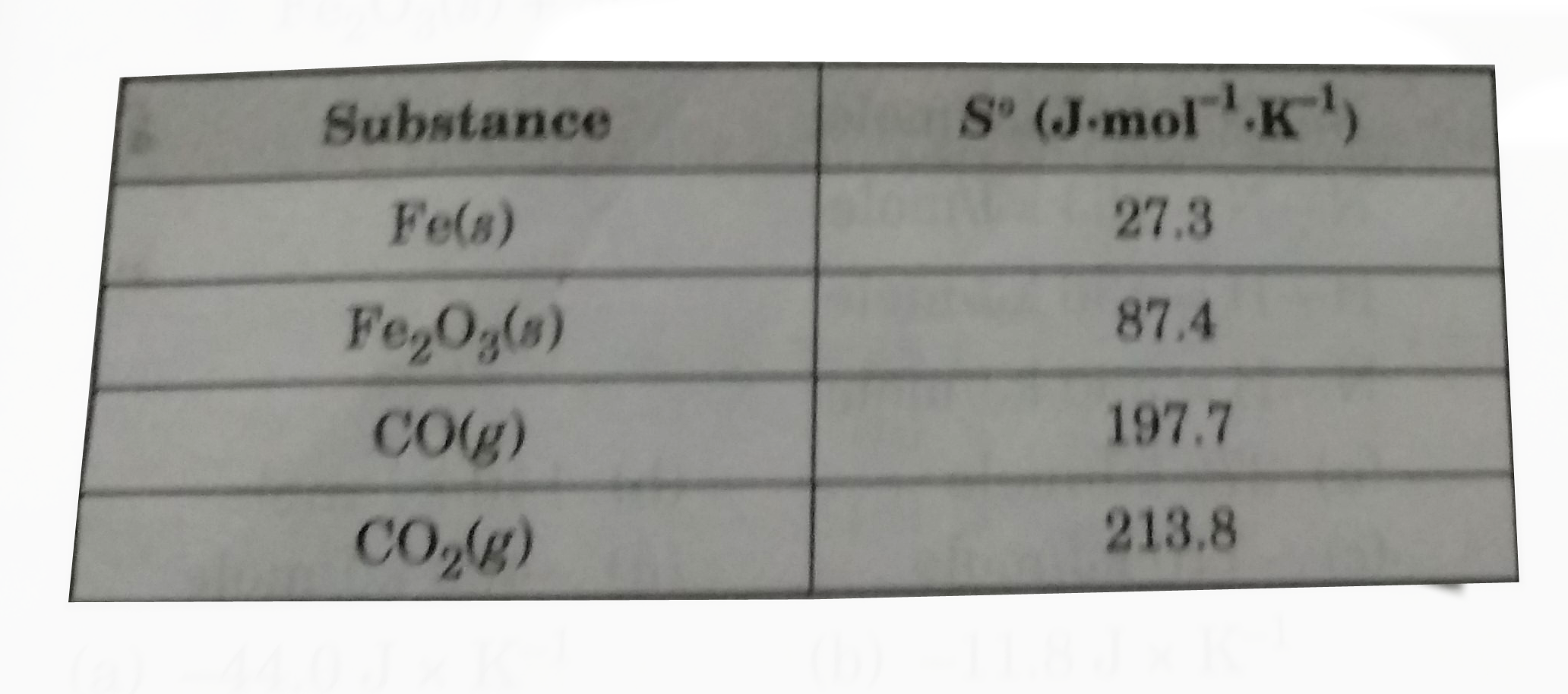Similar Questions
Explore conceptually related problems
Recommended Questions
- What is the value of Delta s^(@) for the reaction below? Fe(2)O(3)(...
Text Solution
|
- What is the value of Delta s^(@) for the reaction below? Fe(2)O(3)(s)+...
Text Solution
|
- Find Delta H(f)^(@) of Fe(2)O(3)(s) if the standard heat reaction Fe(2...
Text Solution
|
- The following teo reaction are known : Fe(2)O(3)(s)+3CO(g)rarr2Fe(s)...
Text Solution
|
- The following two reactionas are known FeO(3)(s)+3CO(g)rarr2Fe(s)+CO...
Text Solution
|
- The following two reactionas are known FeO(3)(s)+3CO(g)rarr2Fe(s)+CO...
Text Solution
|
- निम्न दो अभिक्रियाओं ज्ञात है FeO(s)+3CO(g)to2Fe(s)+3CO(2)(g)," "...
Text Solution
|
- निम्नलिखित अभिक्रयाओं का अपचयोपचय अभिक्रियाओं के रूप में औचित्य स्थापत...
Text Solution
|
- निम्नलिखित अभिक्रियाओं का अपचयोपचय अभिक्रियाओं के रूप में औचित्य स्थाप...
Text Solution
|