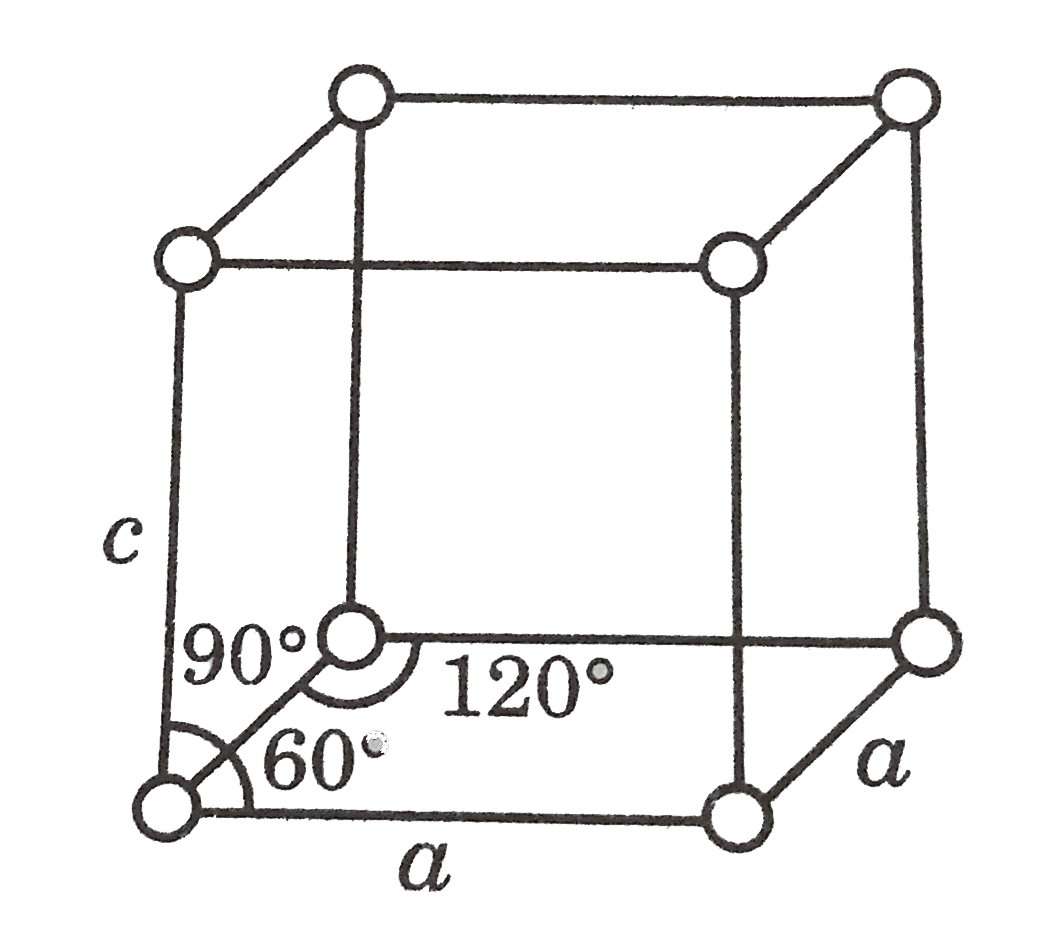
Similar Questions
Explore conceptually related problems
Recommended Questions
- Ice crystallizes in a hexagonal lattice. At the low temperature at whi...
Text Solution
|
- Ice crystallizes in hexagonal lattice. At the low temperature at which...
Text Solution
|
- The lattice parameters of a given crystal are a = 5.62 Å , b = 7.41 Å ...
Text Solution
|
- Ice crystallizes in a hexagonal lattice. At the low temperature at whi...
Text Solution
|
- A(2)B molecules (" molar mass " = 259.8 g//"ml") crystallises in a hex...
Text Solution
|
- Ice crystallizes in a hexagonal lattice. At ascertain low temperature,...
Text Solution
|
- Ice crystallizes in a hexagonal lattice. At the low temperature at whi...
Text Solution
|
- Ice crystallises in a hexagonal lattice having a volume of the unit ce...
Text Solution
|
- Ice crystallises in a hexagonal lattice having a volume of the unit ce...
Text Solution
|