Similar Questions
Explore conceptually related problems
Recommended Questions
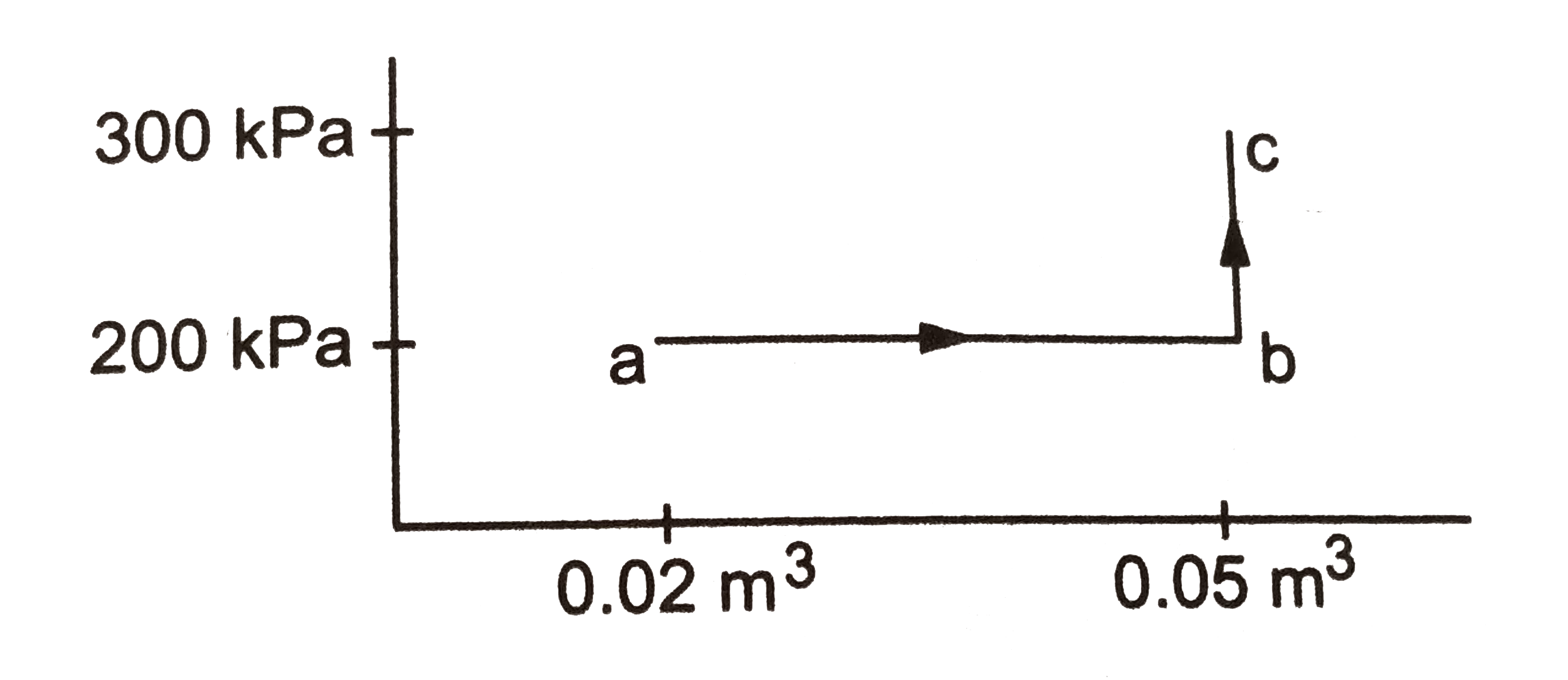
- A substance is taken through the process abc as shown in, if the inte...
Text Solution
|
- A gas is taken through a cyclic process ABCA as shown in, if 2.4 cal o...
Text Solution
|
- A substance is taken through the process abc as shown in, if the inte...
Text Solution
|
- An ideal gas is taken through the process ABC as shown in figure. If t...
Text Solution
|
- एक तन्त्र 50 J ऊष्मा को अवशोषित करता है तथा उस पर 10 J के समतुल्य कार...
Text Solution
|
- In a certain process, 400 cal of heat are supplied to a system and the...
Text Solution
|
- A system absorbs 100 cal of heat and does an external work of 150 J. i...
Text Solution
|
- Calculate the changes in internal energy of a system if, 300 J of ...
Text Solution
|
- In a process, a system loses 125 J of heat when 400 J of work was done...
Text Solution
|