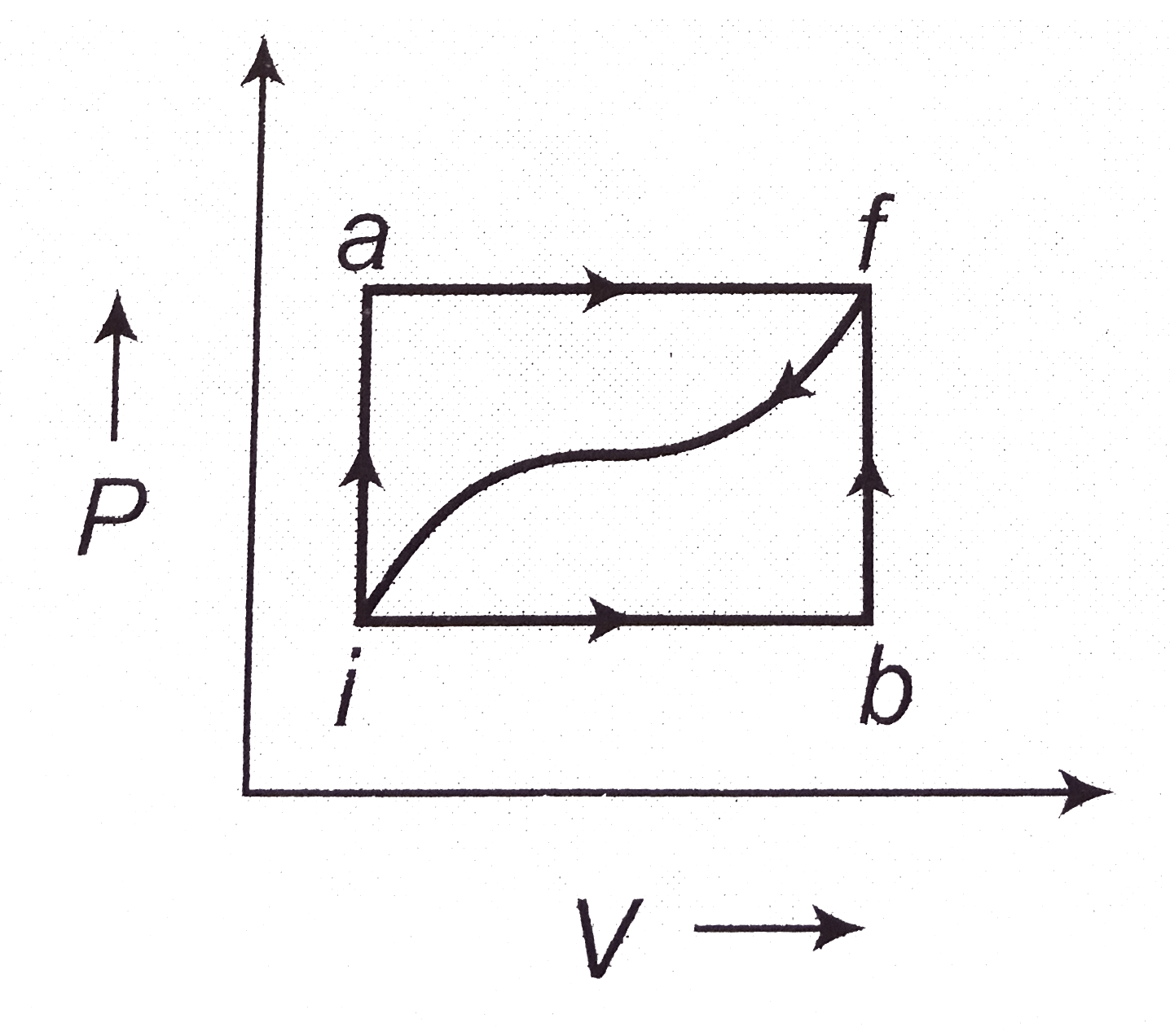Similar Questions
Explore conceptually related problems
Recommended Questions
- When a system is taken from a state i to a state f in Figure, along th...
Text Solution
|
- When a system is taken from state i to state f along the path iaf, it ...
Text Solution
|
- When a thermodynamic system is taken from an initial state I to a fina...
Text Solution
|
- When a system is taken from state f along path iaf, Q = 50 J and W = 2...
Text Solution
|
- An ideal monoatomic gas undergoes the process AB as shown in the figur...
Text Solution
|
- When a system is taken from state i to state f alone the path iaf, it ...
Text Solution
|
- When a system is taken from a state i to a state f in Figure, along th...
Text Solution
|
- A gaseous system undergo a change of state from (1) to (2) by any of t...
Text Solution
|
- When a system is taken from state I to state f along path ia...
Text Solution
|