Similar Questions
Explore conceptually related problems
Recommended Questions
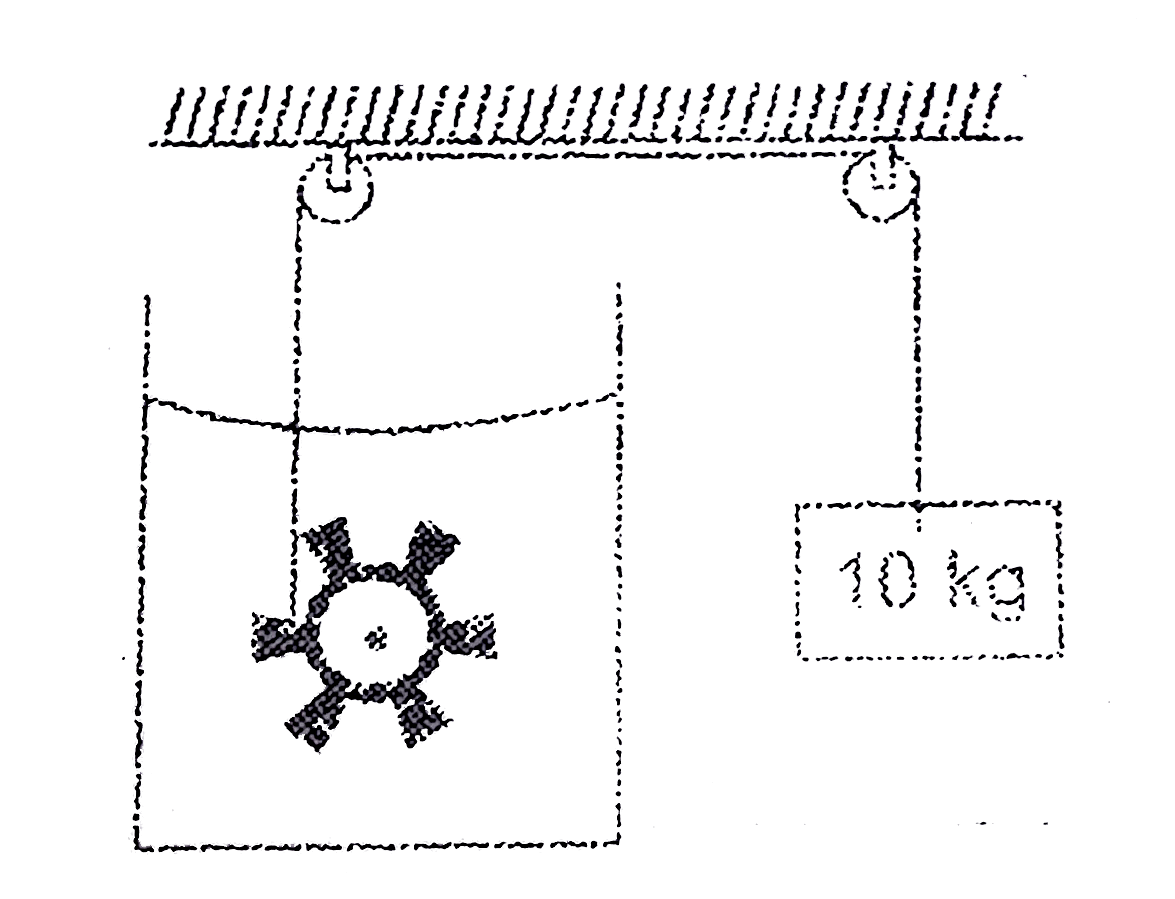
- A paddle wheel is connected with a block of mass 10kg as shown in figu...
Text Solution
|
- Shows a peddle wheel couple to a mass of 12 kg through fixed frictionl...
Text Solution
|
- A closed bottle contains some liquid. The bottle is shaken vigorously ...
Text Solution
|
- As shown in figure a paddle wheel coupled to a mass of 12 kg through f...
Text Solution
|
- A paddle wheel is connected with a block of mass 10kg as shown in figu...
Text Solution
|
- Containers A and B contain a liquid up to same height. They are connec...
Text Solution
|
- Two isotropic blocks A and B are placed as a shown in the figure and a...
Text Solution
|
- Equal masses of two liquids A and B contained in vessels of negligible...
Text Solution
|
- A closed bottle contains some liquid. We shake bottle vigoroulsy for s...
Text Solution
|